Question
Question: How would you make a Ketone from the aldehyde: cyclohexanecarbaldehyde?...
How would you make a Ketone from the aldehyde: cyclohexanecarbaldehyde?
Solution
We are required to make ketone from cyclohexane carbaldehyde. We will do that by adding a carbon so that we will do the oxidation of the product formed to get the ketone. For oxidation we will use a strong oxidising agent. We will see each reaction and understand the process behind it.
Complete answer:
We will start by treating cyclohexane carbaldehyde with Grignard’s reagent the reaction involved is given as
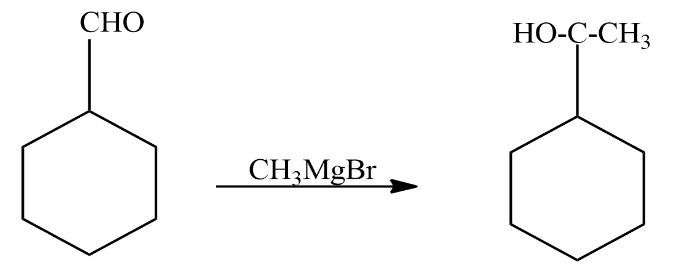
In the above reaction cyclohexane carbaldehyde reacts with Grignard’s agent. It did two things in the reaction: it added a carbon to the reactant and the aldehyde group was converted to alcohol. It formed a secondary alcohol. The compound formed as a product in the above reaction is known as 1−cyclohexylethanol
Now we will treat this product with a strong oxidising agent to oxidise the alcohol group to ketone. the reaction involved is given as
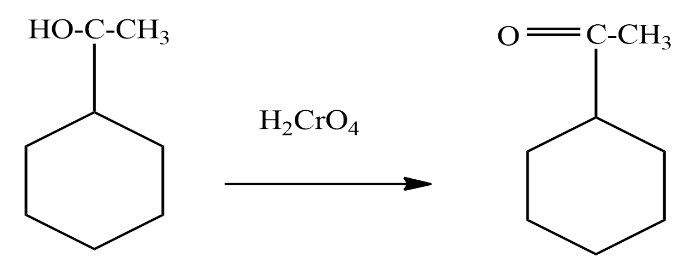
We can see in the above reaction that 1 - cyclohexylethanol is treated with Chromic acid which is a strong oxidising agent to convert the secondary alcohol to ketone. The product formed in the above reaction is known as 1−cyclohexylethanol .
Note:
In the first reaction we are trying to add carbon to cyclohexane carbaldehyde. We are doing so because we want a secondary alcohol as it is necessary for the preparation of ketone also, we are getting an alcohol group so that we can oxidize it to a ketone. In the second reaction Pyridinium chlorochromate can be used instead of Chromic acid for the process of oxidation. It is also known as PCC it is a milder version of chromic acid.
