Question
Question: How would you identify which of the following molecules are chiral and which are achiral: butane, \(...
How would you identify which of the following molecules are chiral and which are achiral: butane, 2−butanol, 2−propanol, 2−bromobutane, and 1−bromobutane?
Solution
Hint : We know that chirality means if a molecule can be superimposed or not when it is mirrored. When we talk about the chiral centre present in a molecule, it is that atom which has four different groups present on each bond in such a way that this makes it a non-superimposable mirror image.
Complete Step By Step Answer:
Chiral centres can also be termed as chirality centres. The two mirror images become non-superimposable. When we talk about the chiral centres, we must know that they have a hybridisation as sp3 present in organic molecules and this is because they can form four bonds. The Cahn-Ingold-Prelog sequence rules assign priorities to the groups attached to each chiral centre.
A chiral molecule can be found when there isn’t any place of symmetry present. One simple example can be our hands. They are the mirror images of each other but when we put them on each other, with both facing the group, they can’t be put on each other. In the options above we can see that:
butane – achiral
- 2−butanol – chiral
- 2−propanol – achiral
- 2−bromobutane chiral
- 1−bromobutane achiral
The reason for the above is that when a molecule has two enantiomeric forms then it is known to be chiral while when it doesn’t have an enantiomer then it is achiral. This can be checked by two methods:
Method one: By drawing the molecule and figuring out if it has a plane of symmetry. Chiral carbons are these carbons which cannot be superimposed on its mirror image by any combination of rotations and transacts. This property of carbon or molecules is called chirality. An achiral molecule is superimposable with its mirror image.
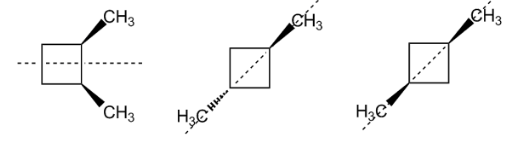
In the above structures, we can see that the plane of symmetry is present.
Methods two: By this can also be done by checking the mirror images of the two molecules. If an imaginary line. Passes through the centre of objects and divides the object so that one half is a mirror image of the other half. Any molecule that has a plane of symmetry is not chiral. If no plane of symmetry is present, then the molecule is chiral.
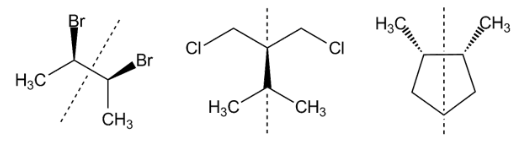
In the above molecules, we can draw a line and then see the mirror images.
Note :
Atoms other than carbon can be chirality centres. When an atom such nitrogen or phosphorus has four different groups or atoms attached to it, it is a chirality centre. The isomerism that is caused by the non-similar arrangements of atoms or functional groups belonging to an atom in space is called stereoisomers.
