Question
Question: How would you identify which carbon is sp, \[s{{p}^{2}}\] and \[s{{p}^{3}}\] ?...
How would you identify which carbon is sp, sp2 and sp3 ?
Solution
The hybridisation is the concept of the mixing of the two orbitals of atoms which has the same energy levels so that they give a degenerated new type of the orbitals. This concept of the intermixing of the atomic orbitals is based on the concept of quantum mechanics. The condition for mixing of two orbitals is that both the orbitals of the atom should have the same energy levels and can be half filled or fully filled.
Complete step by step answer:
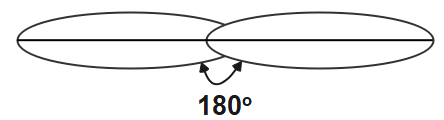
The first type of hybridisation is sp hybridisation. We can identify the sp hybridised carbon atom if the s orbital and the p orbital in the same main shell of the atom is been mixed to form the two new equivalent orbitals. They sp hybridised carbon atom will form the linear molecule with the angle of 180o . It is also known as diagonal hybridisation. In this sp hybridisation the equal amount of s and p character is present that is 50 each.
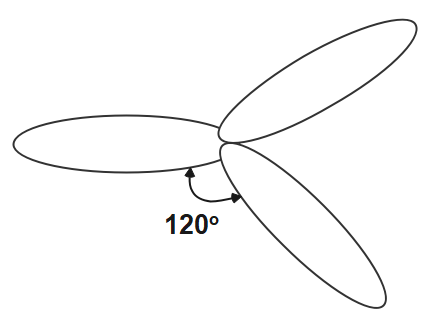
The other hybridisation is sp2 in which we see that one s orbital and two orbitals of p of the same shell of the atom get mix to form the 3 equivalent orbitals. These three orbitals are in one plane thus making the angle of 120o . The s character in this hybridisation is 33.33 and p character is 66.66
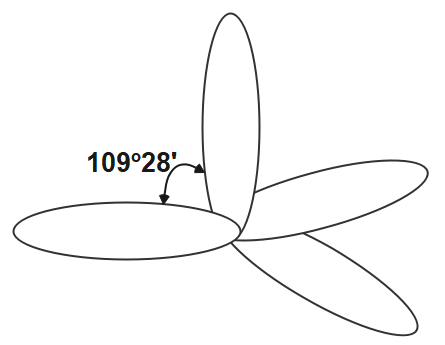
The other hybridisation is sp3 hybridisation. In this hybridisation one orbital of s and three orbitals of p of the same shell of the atom get mixed to form the four new equivalent orbits. In this orbitals the angle which formed between the orbitals is 109o28′ . the s character in this hybridisation is 25 and p character is 75
So if the carbon is sp3 hybridised it will form all single bonds. But the carbon is sp2 hybridised if it forms a double bond. The carbon is sp hybridised if it forms a triple bond. Look at the following examples
CH4 (methane)- in this the carbon is sp3 because it forms four single bonds with the hydrogen atoms.
CH3=CH−CH3 - here the second carbon is sp2 hybridised as it forms double bonds.
CH3=C−CH3 - here the second carbon is sp hybridised as it forms a triple bond.
Note: The orbitals of atoms with the same energies undergo hybridisation. The number of the hybrid orbitals formed is equal to the number of the orbitals of atom mixed. The hybridisation process occurs during the bond formation.
