Question
Question: How would you find the number of diastereomers?...
How would you find the number of diastereomers?
Solution
As we are aware with the diastereomers which are basically those stereoisomers that are non-superimposable as well as are not the mirror images of each other. They also possess the chiral carbons.
Complete step by step answer:
We know that the diastereomers are the chiral carbon containing compounds where chiral carbon is defined as the compound having all the four different groups attached to the central atoms of carbon.
We know that the total number of stereoisomers can be calculated using the formula written as 2n where ‘n’ is the number of chiral carbons or centres present in the compound. Diastereomers are the compounds that possess the same molecular formula but are non-superimposable meaning that the molecules can never be placed similarly on top of one another and they are also non-mirror images of each other. We should also consider the Fischer projection of the stereoisomers which shows that the configuration of the compound is opposite at one or more than one chiral centre.
Therefore, we can say that if any given compound possesses a single chiral carbon centre then it will generally form two diastereomers. If the compound contains a total of two asymmetric carbons or chiral carbons then it will form four diastereomers proven by the above given formula.
For example:
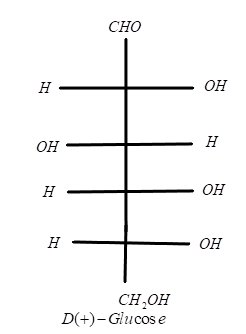
In the case of dextrose (+) glucose, the number of chiral carbons (n) is 4. Using the above formula, we can find out the number of diastereomers.
24=2×2×2×2=16
It means dextrose (+) glucose has 16 diastereomers.
Note: Always remember that the diastereomers are non-superimposable and non-mirror images which is different from enantiomers where enantiomers are those molecules which are actually the mirror images of each other but just like diastereomers, they are also non-superimposable images of each other.
