Question
Question: How would you explain the electrophilic aromatic substitution with a diagram of a reaction mechanism...
How would you explain the electrophilic aromatic substitution with a diagram of a reaction mechanism?
Solution
Hint : Electrophilic substitution reactions as the name suggests occur in systems which are electron rich in nature thus, they cannot occur in cases of normal aliphatic systems i.e., hydrocarbons.
Aromatic systems being electron rich preferably undergo electrophilic substitution reactions.
Complete Step By Step Answer:
We will be showing an electrophilic substitution reaction involving an electrophile E+ and an aromatic reactant i.e., benzene.
The overall reaction is shown below:
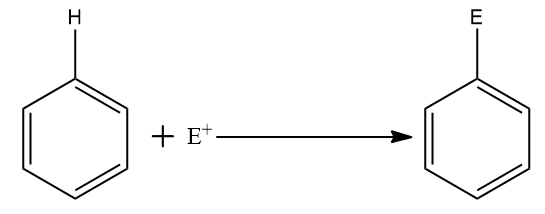
Step one: Generation of the electrophile
This is usually done with the help of a Lewis acid in Friedel craft alkylation and acylation reactions.
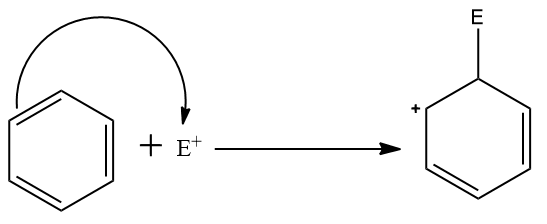
Step two: Attack of the electrophile on the pi- electron system of benzene ring which leads to the formation of a non-aromatic carbocation intermediate.
This is the slowest step in this reaction mechanism as it leads to the loss of aromaticity of the aromatic system.
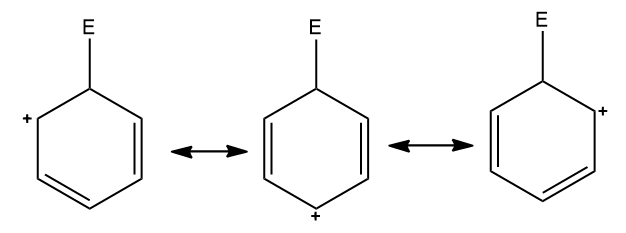
Step three: The positive charge on the non-aromatic carbocation is delocalized throughout the molecule. This is shown below in the three resonating structures.
Step four: The aromaticity of the system is regained by the loss of a proton from the same atom which is bonded to the electrophile.
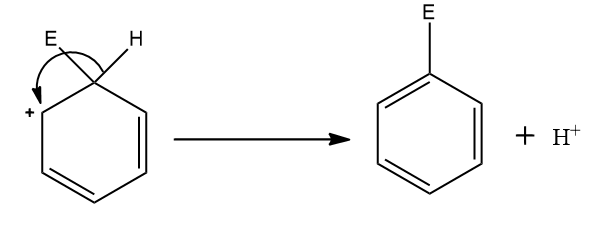
The complete mechanism is shown above.
Note :
During electrophilic substitution reaction, we must be careful about noticing which of the available sites is the most electron-rich and therefore will be the site where the reaction would preferably take place. We must be cautious to notice steric effects too which may change the preferable site of the reaction sometimes.
