Question
Question: How would you draw the Lewis resonance structure of \(C{H_2}Br\)?...
How would you draw the Lewis resonance structure of CH2Br?
Solution
The compound CH2Br is a radical named as bromomethyl radical. The bromomethyl radical does not contain any charge, any double bond. It does not even contain any central atom. The Lewis structure is the representation of the valence electron in a molecule. When the molecule cannot be described by one Lewis structure, then resonance structures are formed which is a set of two or more Lewis structures.
Complete step by step answer:
The given compound is CH2Br, it is a bromomethyl radical.
When the structure of the molecule cannot be represented by one Lewis structure, resonance effect is seen. The resonance structure is the set of two or more Lewis structures that describes the electronic bonding of single polyatomic species.
The bromomethyl radical has only one possible Lewis structure, it does not show any resonance structure. The molecule contains odd number of electrons
The structure of bromomethyl radical is shown below.
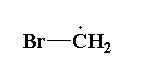
In bromo methyl radical CH2Br, one carbon atom is present, two hydrogen atoms are present and one bromine atom is present.
The valence electrons of carbon is 4.
The valence electrons of hydrogen is (1×2)=2
The valence electrons of bromine is 7
The total electrons are 13.
As the radical contains no charge, no double bonds and bromine is not the central atom, there are no resonance structures formed. Only one Lewis structure is formed.
Note:
The Lewis structure does not explain the geometry of the compound. In the resonance form of the compound, shifting of electrons takes place and not shifting of atoms takes place.
