Question
Question: How would you draw the four possible stereoisomers of \(3 - bromo - 4 - fluorohexane\)...
How would you draw the four possible stereoisomers of 3−bromo−4−fluorohexane
Solution
Start by fischer projection which is the easiest representation of stereoisomers thus assign the vertical chain of carbon as largest number and then try to figure out different positions for fluorine. When you will assign the position to fluorine the alternated 3rd position is to be occupied by another atom to bromine.
Complete step-by-step answer:
Here let’s start by giving an idea that we are talking about stereoisomers, so the difference of positions of atoms is given by the fischer structure. Our first step is to draw a basic skeleton which you see here in the diagram below. So we have taken six carbon atoms on the vertical line while we leave four positions horizontally. These horizontal positions have one fluorine, one bromine and two hydrogens.
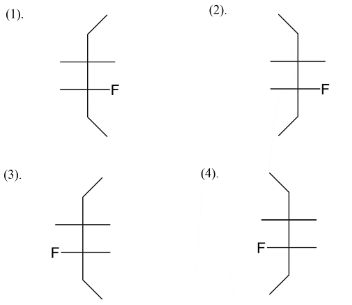
We add fluorine at the C−4 position like in four ways, we get an idea about the four stereoisomers that we will get after assigning positions to bromine also. Next step is to assign bromine as position C−3 alternating right-left-right left and vice versa. So at last we left with fulfilling valences of carbon. So, four stereoisomers of 3−bromo−4−fluorohexane are given above.
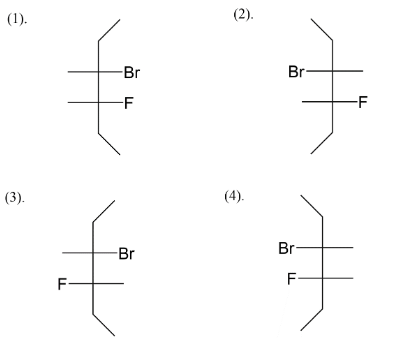
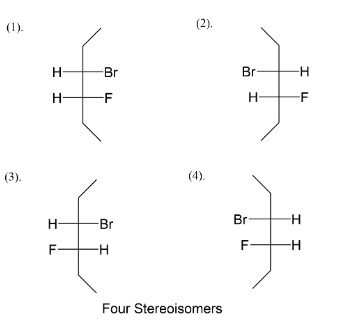
Note: We know there are four types of representation for stereoisomers which are flying wedge, fisher, sawhorse and Newman. Among the four types we usually see fisher structures when it comes to stereoisomers. You have seen the fisher structure in carbohydrates where it was initially thought that all carbohydrates possess fisher structure and gave reaction by it.
