Question
Question: How would you draw constitutional isomers for the molecular formula \({{\text{C}}_{\text{4}}}{{\text...
How would you draw constitutional isomers for the molecular formula C4H8?
Solution
Constitutional isomers are also known by the name of structural isomers and for drawing the constitutional structure of any compound we have to know about the number of atoms and which kind of bond is present in that molecule.
Complete step by step answer:
- Constitutional isomers are those isomers which are formed by the different arrangements of the atoms and bonds present inside the molecule.
-In the question given molecule is C4H8 and it comes under the category of alkene as it matches the general formula of alkene CnH2n where the number of carbon atoms are equal to the number of hydrogen atoms.
-In the given compound all bonds are single only accepting one which is double.
-Constitutional isomers of Butene (C4H8) will be formed by the different arrangement of the atoms and bonds present in it.
-In the first constitutional isomer of Butene (C4H8), double bond is present at the first carbon atom whose name will be But-1-ene and it is showing as CH2 = CH - CH2 - CH3.
-In the second constitutional isomer of Butene (C4H8), double bond is present at the second carbon atom whose name will be But-2-ene and it is showing as CH3 - CH = CH - CH3.
-In the third constitutional isomer of Butene (C4H8), substitution of methyl group is present at the second carbon atom of the propene chain and this isomer is known by the name of 2-methyl propene and it is showing as:
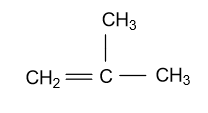
-In the fourth constitutional isomer of butene, it is present in the cyclic form whose name will be cyclo-butane and it is showing as:
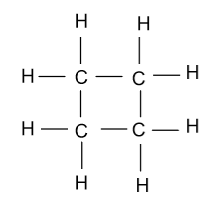
-In the fifth constitutional isomer of butene, it is present in the methyl cyclopropane form which is shown as follow:
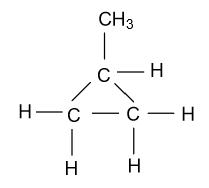
Hence by the above way we will draw the constitutional isomers for the molecular formula C4H8.
Note: Here some of you may think that how cyclic structure of butene is possible as in the butene double bond is present but in the cyclic structure only single bonds, so the reason is that in constitutional type of bond will be changed but number of atoms always remain constant.
