Question
Question: How would you draw an enthalpy diagram for: \({N_2}(g) + 3{H_2}(g) \to 2N{H_3}(g){\text{ }}\Delta ...
How would you draw an enthalpy diagram for:
N2(g)+3H2(g)→2NH3(g) ΔH=−100.3kJ?
Solution
The enthalpy is defined as the heat change of a reaction. It contains both a positive and a negative sign. The positive sign indicates that energy is absorbed and the negative sign indicates that energy is released.
Complete step by step answer:
The given reaction is the reaction of nitrogen gas and hydrogen gas to generate ammonia gas. The heat of the reaction, i.e. ΔH contains a negative sign which shows that the energy is released in the reaction. It is also a product of the reaction. The reaction is written as
N2(g)+3H2(g)→2NH3(g)+heat
The production of heat in a reaction is termed as an exothermic reaction according to the principle of thermochemistry. In case of exothermic reaction as energy is liberated or released in the form of heat the energy of the product is lower than that of reactants.
For the given reaction one mole of nitrogen gas combines with three moles of hydrogen gas to produce one mole of ammonia. In this process heat is liberated to the surroundings. This heat on the right hand side of the equation is indicative of the fact that it balances both sides of the equation.
This means that the energy of the product ammonia is lower than that of the reactants. During the reaction an amount of energy is required to be added to the system which is known as the activation energy. Also a stage is reached where the reactants and products coexist which is called as the transition state. The energy equal to the activation energy is required to overcome the transition state and make the reaction go to completion.
The enthalpy diagram for the given conversion is shown as:
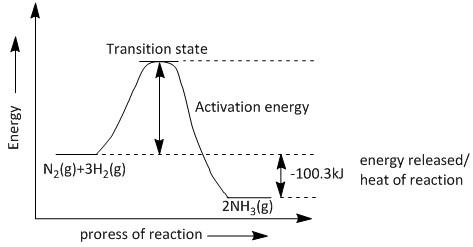
Note:
Unlike exothermic reactions where energy is released, energy is absorbed in endothermic reactions. The heat of the reaction carries a positive sign as the energy is absorbed by the reactants to begin the reaction. In this case the energy of the reactants is lower than that of products.
