Question
Question: How would you describe the arrangement of sodium ions \(N{a^ + }\)and chloride ions \(C{l^ - }\)in a...
How would you describe the arrangement of sodium ions Na+and chloride ions Cl−in a crystal of sodium chloride NaCl?
Solution
Take help of types of unit cell, which are of three types simple cubic, body centred and face centred. These unit cells are a small portion of three dimensional lattice, when we rotate the unit cells all around it will give us a lattice. Here ions are arranged in such a way that crystals of sodium chloride NaCl have FCC type of arrangement.
Complete step-by-step answer:
In the chapter on solid state, it gives us an idea of how the complex structures are arranged. A lattice is formed by the number of atoms arranged in a small unit cell. Unit cells have sides which may be equal or not. Famously we talk a lot about the cubic cell type. These cells are such that the position of atoms or ions matters. We have three types of cubic unit cells. The first one is the simple cubic in this type. The particles are just at the corner and you can imagine that while sitting in your room. So as there are eight corners in a cube so there are eight atoms in a simple cubic unit cell.
In the other two that are body centred and face centred, the atoms are arranged including eight corners and also at the body centred which is just between the cube and in face centred atoms are also present at each of the face including the corners.
The lattice crystal given of sodium chloride NaCl , a salt which we used for cooking, has FCC face centred crystal lattice and contains a unit cell in which there are atoms at the corners and also at each face. In the unit cell there are voids which are present, in which the particles take its site. So in sodium chloride NaCl crystal.
We have a lattice which is formed by chloride ions Cl− present at the corners and sodium ions which are present in the voids. Imagine such that the chloride ions Cl− which are bigger in size are present at the corners of a cube and all other sodium ions Na+ are at each face centred site.
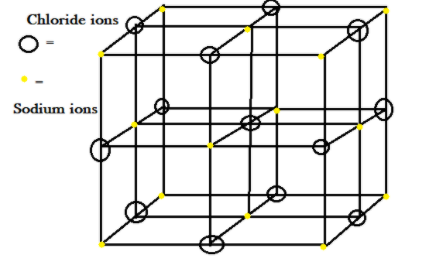
Note: To understand any crystal lattice, consider a small part i.e. unit cell where the atoms are arranged. In the above example of NaCl, why chloride ions Cl− formed lattice and all sodium ions Na+ are present at a smaller void site? This arrangement is possible and decided by the radius ratio rule, thus we can say cation decides that in which void it wants to live formed by arrangement of anions.
