Question
Question: How would you compare and contrast the structures of butanes and butanol?...
How would you compare and contrast the structures of butanes and butanol?
Solution
In organic chemistry, hydrocarbons are an important topic. The hydrocarbons are majorly classified as three groups. There are alkane, alkene and alkyne. The alkane means carbon-carbon single bond. The alkene has a carbon-carbon double bond. The alkyne means carbon-carbon having triple bond in the molecule. It has some general formulas. The general formula of alkane is CnH2n + 2. The general formula of alkene is CnH2n. The general formula of alkyne is CnH2n - 2.
Complete answer:
The word compare means similarity between the two things.
The word contrast means difference between the two things.
The molecular formula of butane is
CH3 - CH2 - CH2 - CH3.
The molecular formula of butanol is
CH3 - CH2 - CH2 - CH2OH.
The structural formula of butane is
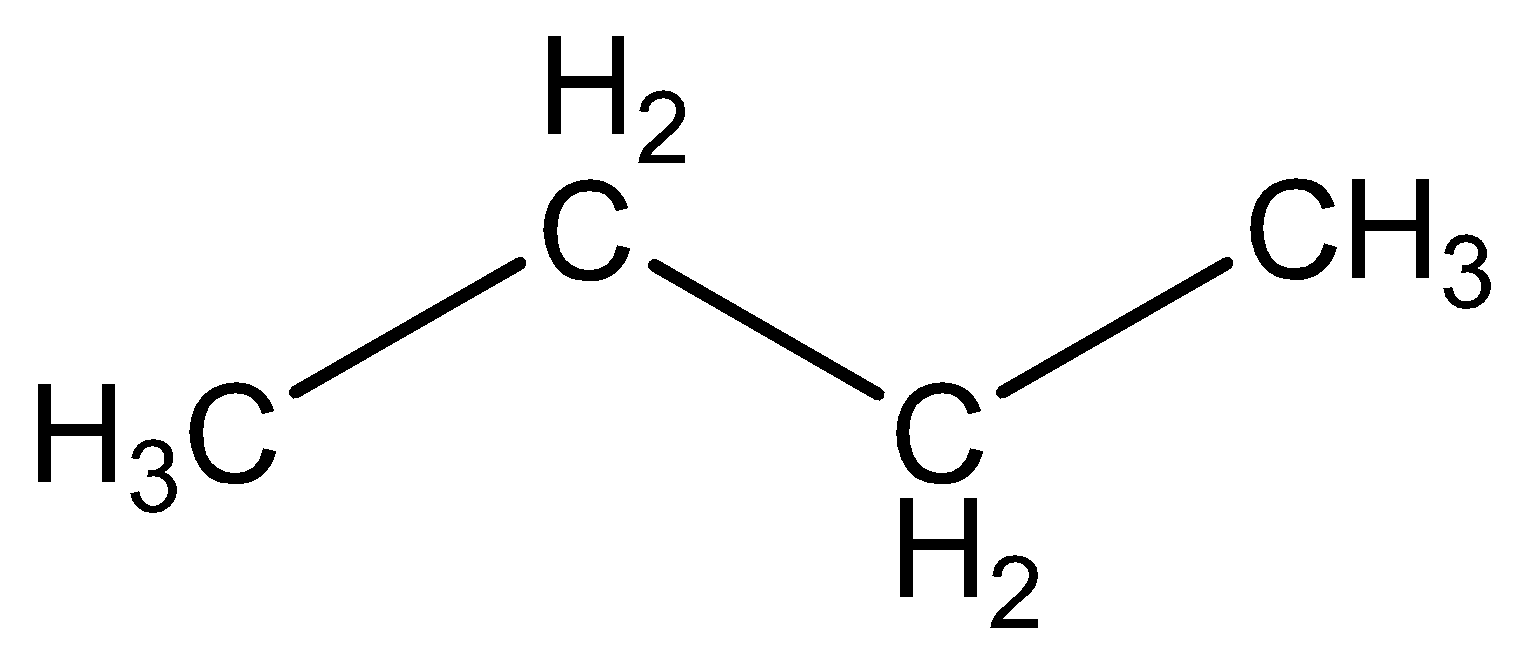
The structural formula of butanol is
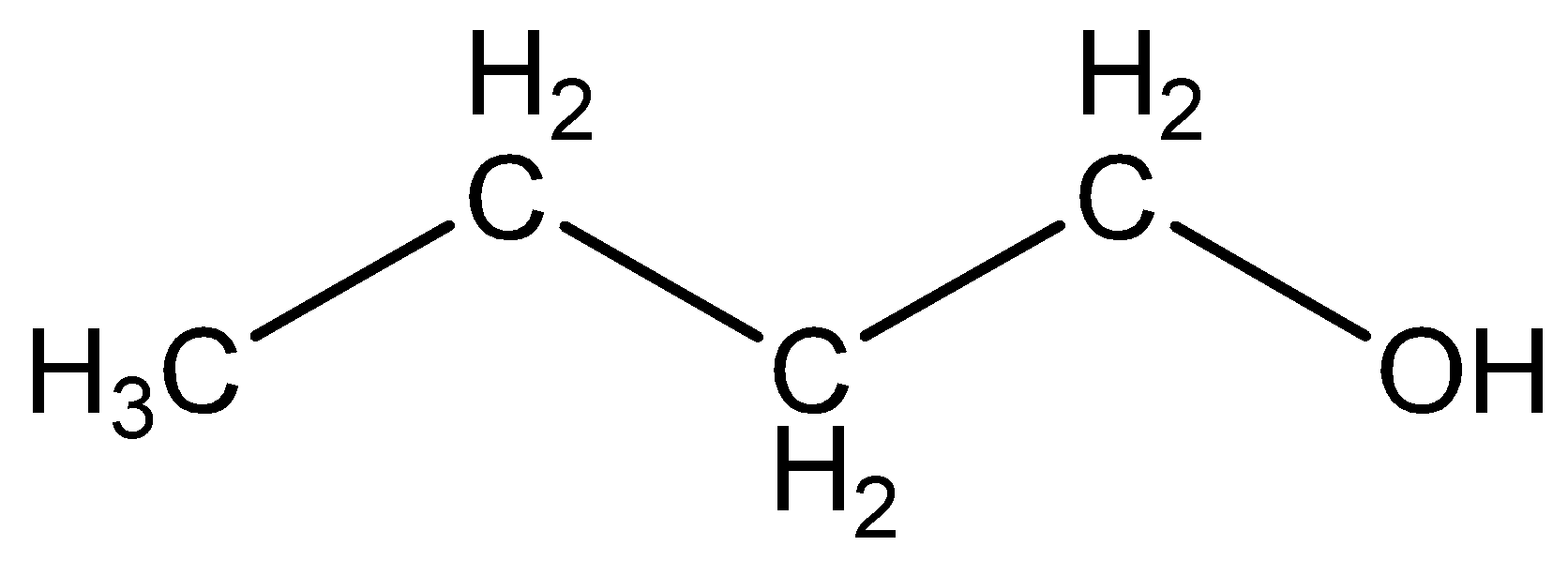
Comparing the structures of butanes and butanol, both have four carbon atoms and ten hydrogen atoms in the structure.
Contrast the structures of butanes and butanol is butanol have oxygen atom in structure but that oxygen atom is not there in butane.
Note:
In chemistry two reactions are mainly organic. The two reactions are oxidation and reduction reaction. The oxidation reaction is nothing but in chemical reaction from reactant to product the addition of oxygen or removal of hydrogen or gain of electron in the product. The reduction reaction is nothing but in chemical reaction from reactant to produce the addition of hydrogen or removal of oxygen or loss of electrons. The oxidation or reduction reaction plays a key role for converting one functional group to another functional group in the organic chemical reaction.
