Question
Question: How would I determine the correct Lewis structure for \(NO_{3}^{-}\)?...
How would I determine the correct Lewis structure for NO3−?
Solution
Hint The lewis dot structure tells the number of valence electrons around the symbol of the element. The number of valence electrons in the nitrogen is 5 and the number of valence electrons is 6, and one bond is formed by two electrons in which one-one electrons are donated by each atom.
Complete step by step answer:
The lewis dot structure tells the number of valence electrons around the symbol of the element. According to the number of electrons in the atoms are arranged in the shells and the last shell of the atom is the valence shell and the electrons in that shell are valence electrons and these valence electrons are responsible for the bond formation.
The number of valence electrons in the nitrogen is 5 and the number of valence electrons is 6, and one bond is formed by two electrons in which one-one electrons are donated by each atom. The bonds must be formed in such a way that each atom can complete its octet.
There must be five dots around the nitrogen atom and there must be six electrons around the oxygen atom.
NO3− has one nitrogen atom and three oxygen atoms so, the total electrons in the Lewis dot structure will be:
5+6+6+6+1=24
The negative charge means there is the addition of one electron in the compound.
So, the structure is given below:
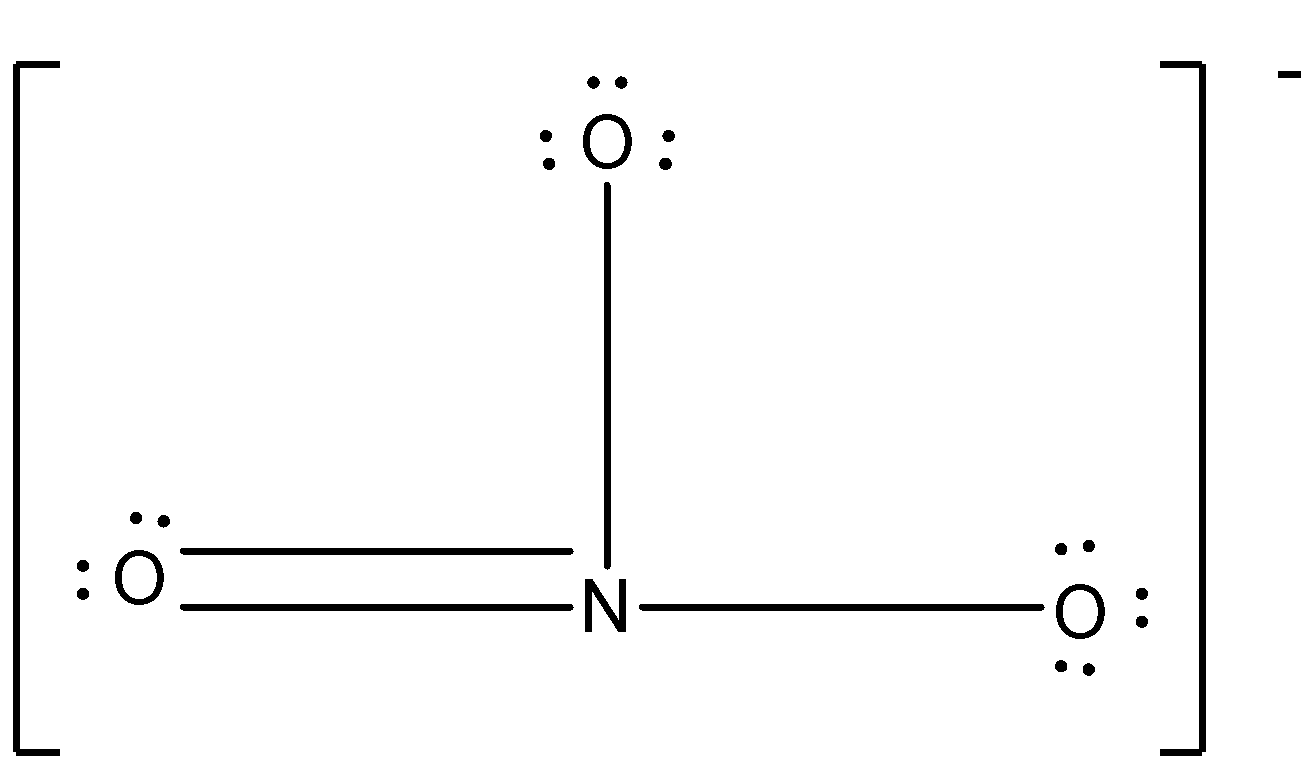
Note: If the overall charge on the molecule is negative then the electron is added to the molecule and if the overall charge on the molecule is positive then the electrons are deducted from the molecule. As the charge increases the number of electrons gained or lost also increases.
