Question
Question: How will you separate oil and water from their mixture?...
How will you separate oil and water from their mixture?
Solution
Hint : More often the substances that we see around us are not in their pure form. They are fundamentally a combination of at least two substances. Interestingly, combinations will in general additionally come in various forms. Therefore, there are a few sorts of separation methods that are utilized in isolating a combination of substances. With respect to the requirement for isolation, it is generally done to eliminate every one of the impure materials and acquire useful parts.
Complete Step By Step Answer:
Two immiscible fluids, oil, and water can be isolated by utilizing Separating Funnel. The combination of oil and water structures two separate layers since they are totally insoluble in one another. Oil frames the upper layer while water shapes the lower. In an isolating pipe they are saved for resting, when two layers become steady by utilizing the isolating channel they are filtered individually.
When the fluids are taken in the funnel, we can see the formation of two layers. The denser fluid sinks to the base and the other fluid is on the top. A conical flask is set at the base to collect the denser fluid. The valve permits control of when and how the fluid is let through down to the conical flask.
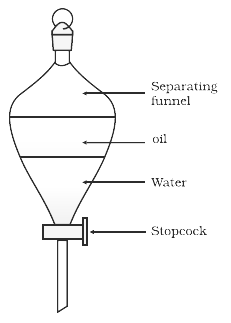
Separating funnel
Note :
The process includes utilizing the combinations of inconsistent molecule density. The process includes exploiting the inconsistent thickness of the particles in the mixture. Since water is denser than oil, it tends to be isolated through the channel and left in the pipe with an oil layer.
