Question
Question: How will you prepare methoxy methane from (A) Methyl bromide (B) Diazomethane (C) Methyl alcoh...
How will you prepare methoxy methane from
(A) Methyl bromide
(B) Diazomethane
(C) Methyl alcohol
Solution
Methoxy methane (dimethyl ether) is the organic compound with the formula CH3OCH3, simplified to C2H6O. The simplest ether, it is a colourless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. It is an isomer of ethanol.
Complete step by step solution:
We have been provided methyl bromide, diazomethane and methyl alcohol.
We need to obtain methoxy methane (dimethyl ether) from these three compounds,
So, firstly we have methyl bromide: CH3OH CH3Br
For converting into methoxy methane we will be treating methyl bromide with dry ether along with NaOCH3,
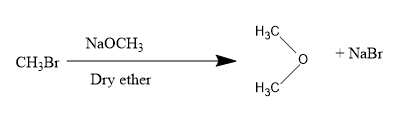
Next, we have diazomethane: N≡N−CH2
For, converting into methoxy methane, add the ethyl alcohol along with the diazomethane. By treating the ethyl alcohol with the diazomethane in the presence of the fluoroboric acid, methoxy methane will be formed,
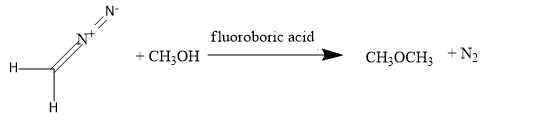
Next, we have methyl alcohol: CH3OH,
For converting into methoxy methane, diazomethane is added in methyl alcohol in the presence of fluoroboric acid,
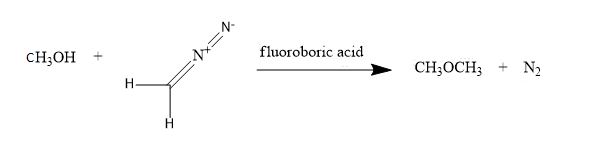
So, these are the conversions of methyl bromide, diazomethane and methyl alcohol into methoxy methane.
Note: It is used as an aerosol propellant, as a refrigerant, and as a blowing agent for the production of some foams. It can also be used as a fuel in diesel engines. Ethyl methyl ether, or methoxy ethane, is a colourless gas at room temperature, having a boiling point of 7.6 degree Celsius. Like dimethyl ether, it is fairly water soluble.
