Question
Question: How will you prepare chlorobenzene from benzene diazonium chloride?...
How will you prepare chlorobenzene from benzene diazonium chloride?
Solution
We know that when a diazonium salt is treated with copper (I)chloride (Cu2Cl2) or copper (I) bromide (Cu2Br2), the corresponding haloarene is formed. This reaction is known as the Sandmeyer reaction. It is used for introducing a chloro or bromo group in the benzene ring.
Complete step by step answer:
According to the question we have to state how chlorobenzene will be prepared from benzene diazonium chloride by the Sandmeyer reaction.
Hence , The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. This reaction is named after the Swiss chemist Traugott Sandmeyer.
The Sandmeyer reaction is a method for substitution of an aromatic amino group by preparation of its diazonium salt followed by its displacement with a nucleophile, often catalyzed by copper (I) salts. The nucleophile can include halide anions, cyanide , thiols, water and others.
The substitution of an aromatic amino group is possible by preparation of its diazonium salt and successively displacement with a nucleophile. General reaction equation of formation of diazonium salt and haloarenes given below:
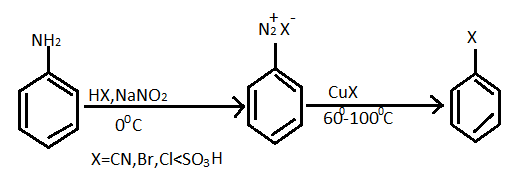
For the given question, reaction of preparation of chlorobenzene from diazonium chloride as follows :
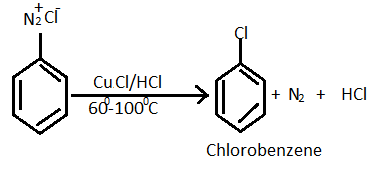
Note: Benzene diazonium salt is formed by treating an aromatic primary amine with NaNO2 and dil. HCl at low temperature. The process is known as diazotization. Diazonium salts are highly reactive compounds. They are used in the preparation of a large number of arene derivatives. The Sandmeyer reaction helps us to provide a method through which we can perform unique transformations on benzene for example halogenation, cyanation , sulphonation and hydroxylation.
