Question
Question: How will you prepare benzoic acid from benzene, with the help of Friedel-Craft reaction? Also, write...
How will you prepare benzoic acid from benzene, with the help of Friedel-Craft reaction? Also, write any two chemical properties of Benzoic acid?
Solution
Benzoic acid can be prepared from the benzene with the help of the Friedel-Craft reaction. In the case of benzoic acid, the acyl group is substituted into the benzene ring. This reaction is also known as Friedel-Crafts acylation of Benzene.
Complete step by step answer:
The benzene ring is reacted with a mixture of carbonyl chloride COCl2 and AlCl3 (aluminum chloride) that acts as a catalyst. The product benzoyl chloride is formed.
The reaction is as follows:
C6H6+COCl2→C6H5COCl
Or
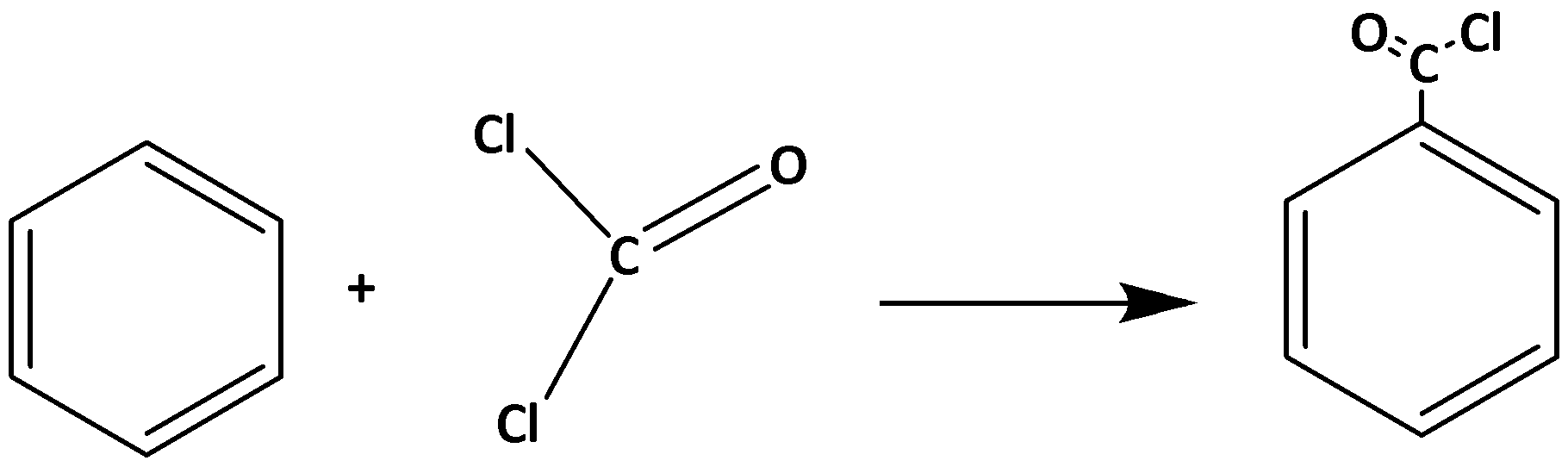
This reaction is an example of an electrophilic substitution reaction. The electrophile is ClCO+ that is produced by the reaction of carbonyl chloride and aluminum chloride.
The reaction is as follows:
COCl2+AlCl3→ClCO++AlCl4−
The electrophilic substitution mechanism is divided into 2 stages:
Stage 1 –
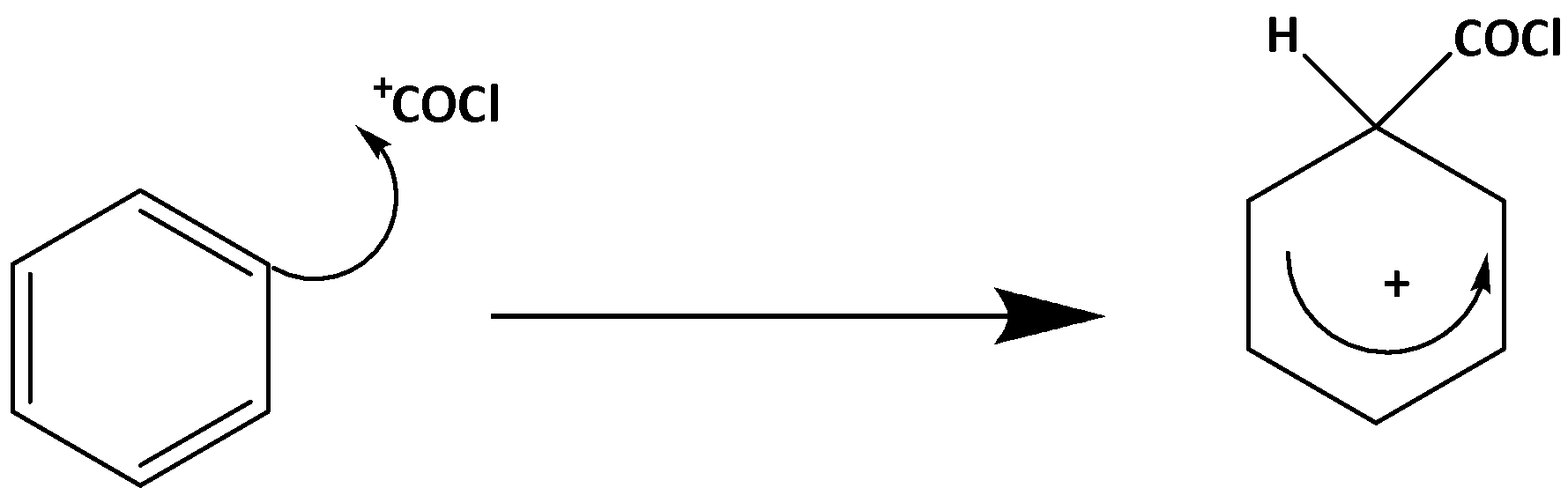
Stage 2 –
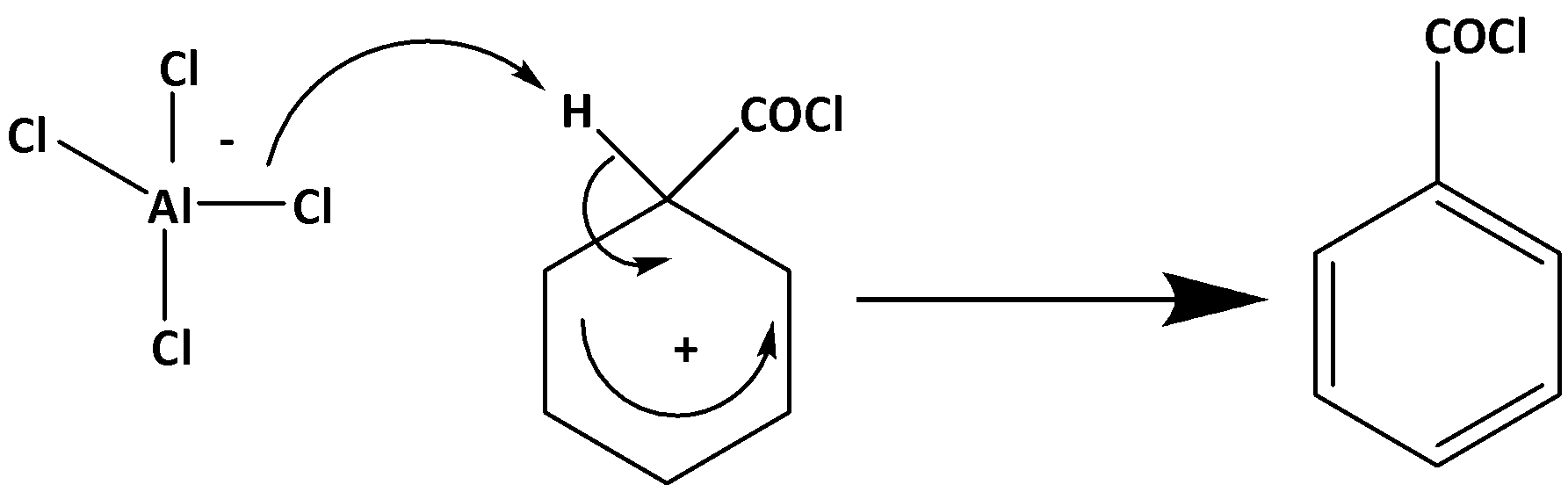
Now when this product is hydrolyzed in presence of HCl. benzoin acid is formed as follows,
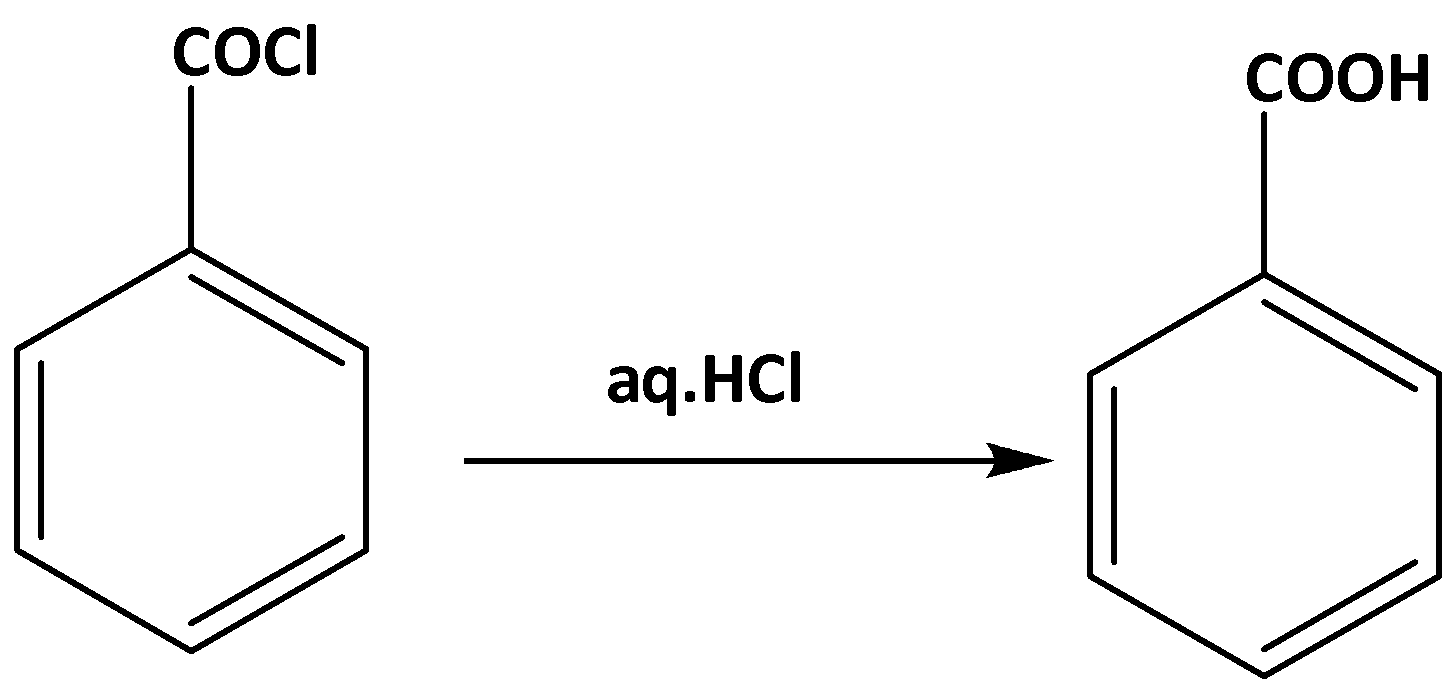
Some chemical properties of benzoic acid are,
Benzoic acid can be converted into benzyl alcohol by treating it with lithium aluminum hydride.
The reaction is shown below,
C6H5COOH+LiAlH4→C6H5CH2OH
When benzoic acid reacts with thionyl chloride benzoyl chloride is formed as follows,
C6H5COOH+SOCl2pyridineC6H5COCl+SO2+HCl
Note: A student can get confused between acylation and alkylation. Alkylation is the transfer of an alkyl group from one compound to another.
To convert benzene to ethylbenzene there is a very well-known reaction which is the Friedel-Craft alkylation reaction. In presence of aq. AlCl3 it reduces the nucleophilicity of the benzene ring by the formation of the cation of the benzene ring. the reaction is shown below,
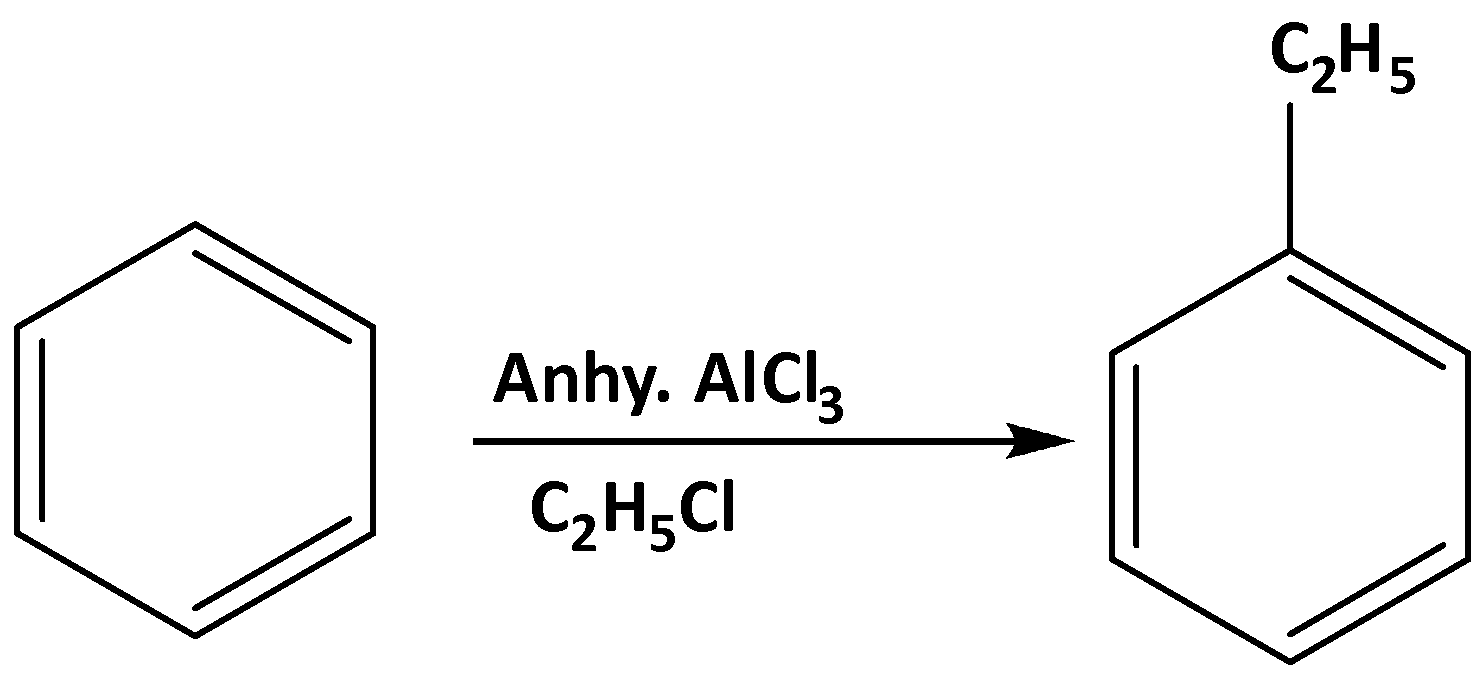
A student can also get confused between electrophilic substitution and nucleophilic substitution. Electrophilic substitution involves the displacement of a functional group by an electrophile, i.e., hydrogen whereas nucleophilic substitution involves the attack of a positively charged atom by a nucleophile.
