Question
Question: How will you prepare 2-methyl-propan-2-ol from methyl magnesium bromide? Give a balanced reaction fo...
How will you prepare 2-methyl-propan-2-ol from methyl magnesium bromide? Give a balanced reaction for it.
Solution
Methyl magnesium bromide is a simplest organo-metallic, Grignard reagent which is higher flammable, colourless and moisture sensitive and is usually available as a solution in tetrahydrofuran.
Complete step by step solution:
-Grignard reagent is a chemical compound with the generic formula R-Mg-X, where X is a halogen, and R is an organic group, generally an alkyl or aryl. Magnesium bromide is among two typical examples of the Grignard reagent with chemical formula as Cl−Mg−CH3 and another being phenylmagnesium bromide with chemical formula as (C6H5)−Mg−Br.
-Grignard reagents are popularly known in organic synthesis for creating new carbon-carbon bonds. The main reacting group in Grignard’s reagent is alkyl or aryl part.
-As we know that carbonyl group-containing compounds, when reacted with Grignard’s reagent, get converted to alcohol compounds. Conversion of magnesium bromide to 2-methyl-propan-2-ol can be done in the following steps-
(i) Let us assume the carbonyl compound is Ethanal which in the first step reacts with Grignard’s reagent in the following manner.
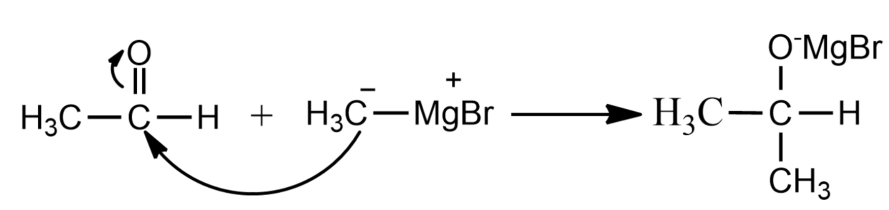
(ii) In the next step, the adduct formed in the first step is now hydrolysed to form 2-methyl-propan-2-ol.
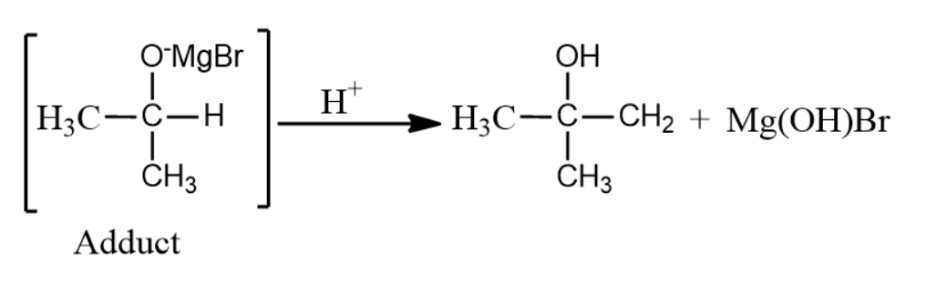
Note: 2-methyl-propan-2-ol, also called Tert-Butyl-alcohol is the simplest tertiary alcohol and is one among the four isomers of butanol. It is more unique than other isomers of butanol because it tends to be solid at room temperature and has a camphor-like odour. It is miscible in water, ethanol and diethyl ether. It is used as a solvent, ethanol denaturant, paint remover ingredient, and gasoline octane booster. This compound is naturally found in guava and ginger.
