Question
Question: How will you obtain A. Diacetone amine from acetone B. Urotropine from formaldehyde C. Acetald...
How will you obtain
A. Diacetone amine from acetone
B. Urotropine from formaldehyde
C. Acetaldoxime from acetaldehyde
Solution
We should know the structure of the acetone, formaldehyde and acetaldehyde to prepare the diacetone, urotropine and acetaldoxime. The structures of acetone, formaldehyde and acetaldehyde are as follows.
ACETONECH3COCH3,FORMALDEHYDEHCHO,ACETALDEHYDECH3CHO
Complete answer:
- In the question it is given to write the preparation of diacetone amine from acetone, urotropine from formaldehyde and acetaldoxime and acetaldehyde.
a) The preparation of diacetone amine from acetone is as follows.
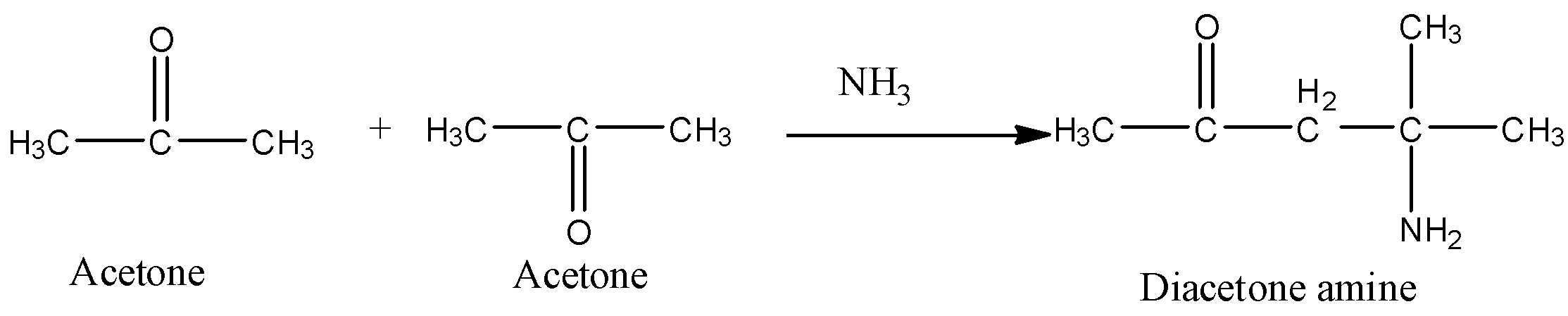
- In the above chemical reaction two moles of acetone are going to react with one mole of ammonia and form one mole of diacetone amine as the product.
- The diacetone amine is going to form by the condensation of 2 moles of acetone with ammonia.
b) The preparation of Urotropine from formaldehyde is as follows.
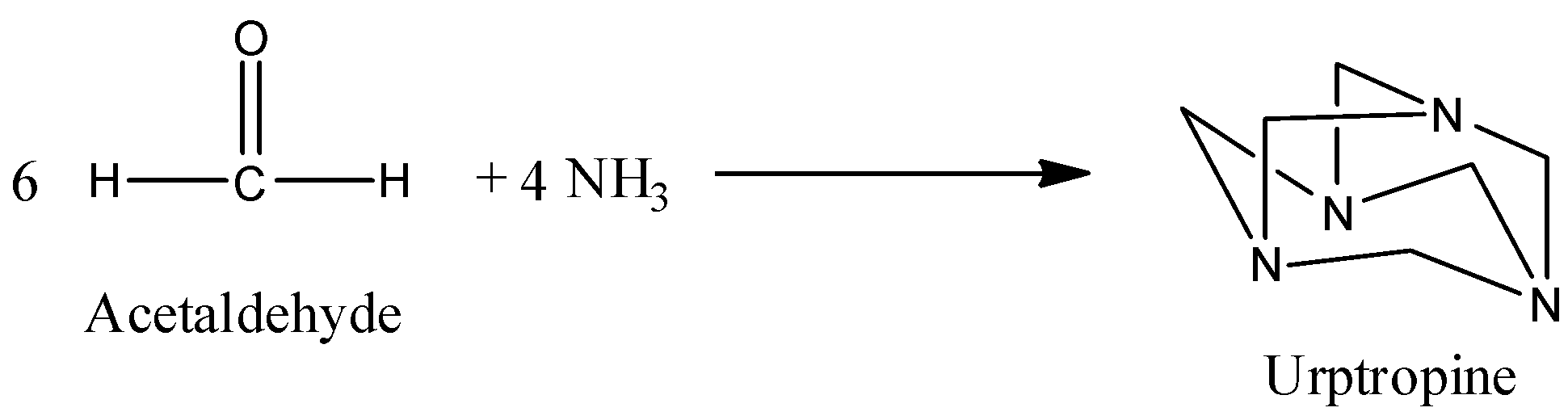
- In the above chemical reaction 6 moles of formaldehyde reacts with four moles of ammonia and forms one mole of urotropine as the product.
c) The preparation of Acetaldoxime from acetaldehyde is as follows.
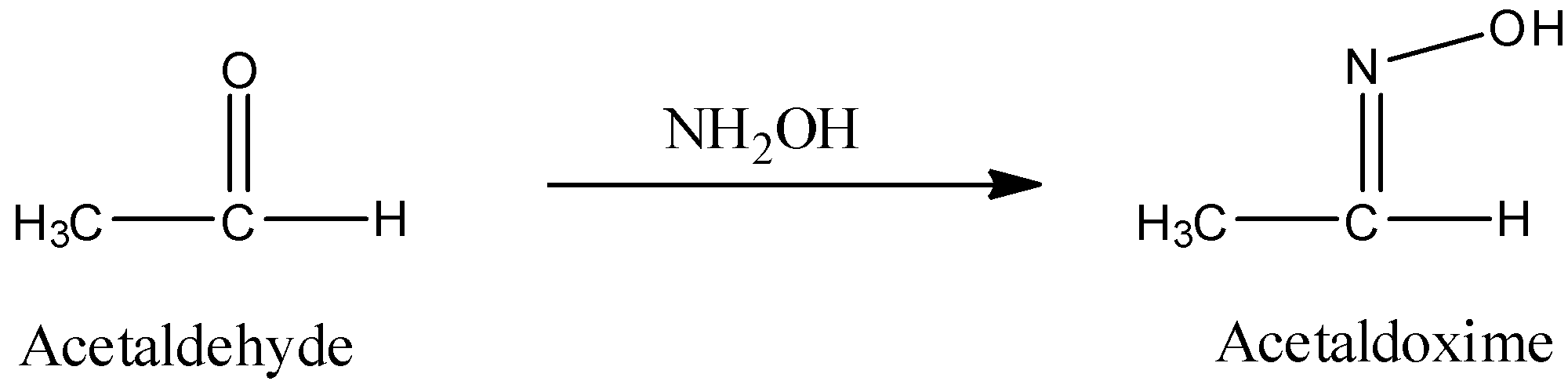
- In the above chemical reaction one mole of acetaldehyde is going to react with hydroxyl amine and forms one mole of the acetaldoxime as the product.
Note:
Methenamine is also called urotropine and it is a cage like structure. Urotropine is going to look like adamantine. The salt form of methanamine is going to be used to treat the urinary tract infection disease. Acetaldoxime is useful in boilers to act as an oxygen scavenger.
