Question
Question: How will you convert the following? Chlorobenzene to D.D.T...
How will you convert the following?
Chlorobenzene to D.D.T
Solution
Chlorobenzene is an organic molecule in which one hydrogen atom of the benzene ring is substituted with the chlorine atom and D.D.T is known as 2,2-bis(4-chlorophenyl)-1, 1, 1-trichloroethane. We get D.D.T, when the chlorobenzene is treated with chloral.
Complete step by step answer:
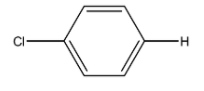
Chlorobenzene is an organic compound in which the chlorine atom is attached to one of the carbon atoms in the benzene ring. The formula is C6H5Cl and the structure is given below:
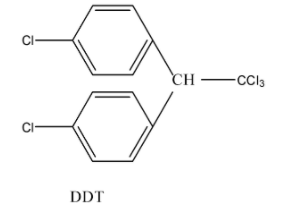
D.D.T is an organic compound which is known as p,p’-DichlorodiphenylTrichloroethane is a misnomer. Its actual name is 2,2-bis(4-chlorophenyl)-1, 1, 1-trichloroethane. Its formula is C14H9Cl5 and the structure is given below:
DDT was first prepared in 1873 but it was not known until 1939. In 1939 Paul Muller, at Geigy pharmaceutical in Switzerland, discovered the DDT as an effective insecticide.
The chlorobenzene is heated with chloral in the presence of sulfuric acid gives DDT. The chloral is trichloroacetaldehyde. In this reaction, 2 moles of chlorobenzene is required. The reaction is given below:
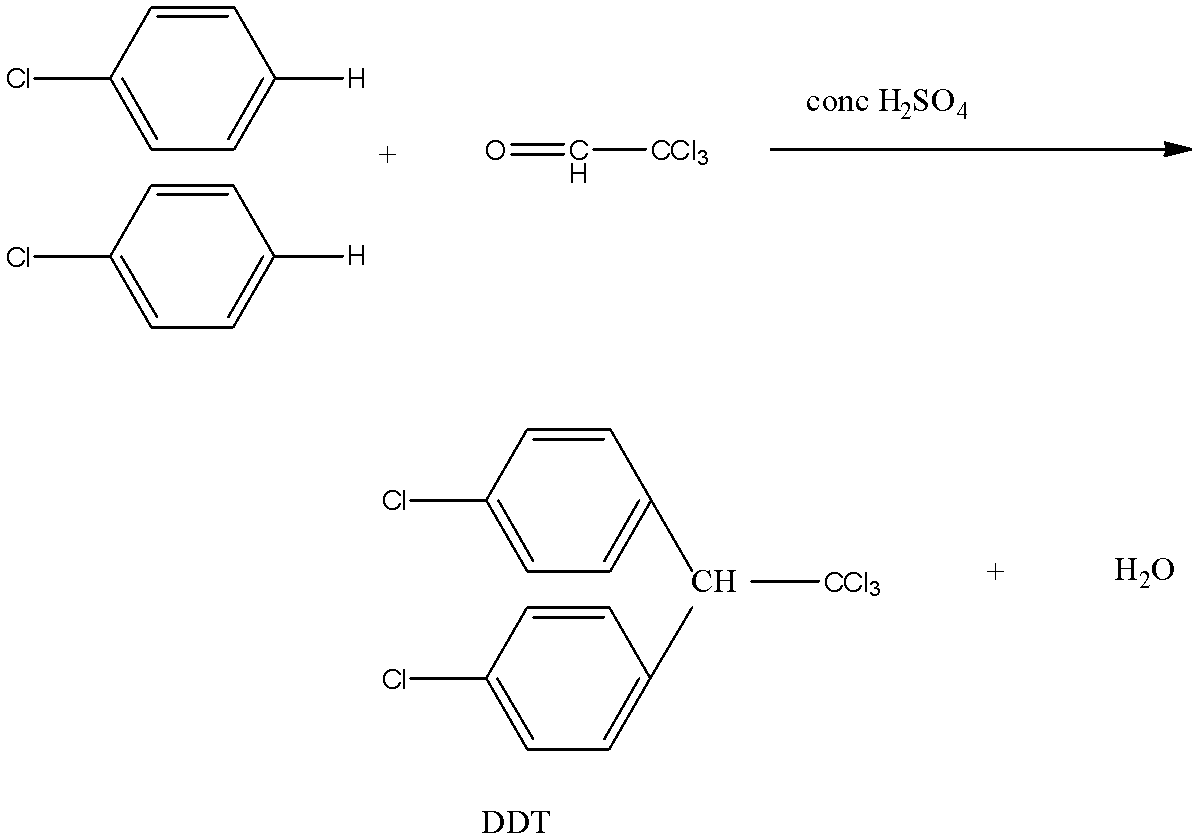
This compound is formed by the elimination of a water molecule.
DDT is used as an insecticide because it is cheap and powerful. It is mostly used in sugarcane and fodder crops and to kill insects and mosquitoes. It is particularly used to the Anopheles Mosquitoes which spread malaria and lice that carry typhus.
Note: The use of DDT enormously increases after World War II. But in the late 1940s, the problem of the use of DDT started arising, because the insects with tolerance to DDT were developing.
