Question
Question: How will you convert the Ethanol to formaldehyde?...
How will you convert the Ethanol to formaldehyde?
Solution
Ethanol is an organic compound in which there are two carbon atoms and the functional group −OH is present. Formaldehyde is a compound having one carbon atom having the functional group −CHO. So, the two carbon atoms have to be converted into one carbon atom.
Complete step by step solution:
Ethanol is an organic compound in which there are two carbon atoms and the functional group −OH is present and its formula is CH3−CH2OH.
Formaldehyde is a compound having one carbon atom having the functional group −CHO and its formula is HCHO.
So conversion of ethanol to formaldehyde, there are three steps involved. First, we have to convert the ethanol to ethene. And then this ethene is converted into formaldehyde.
The ethanol can be converted into ethene by the action of concentrated sulfuric acid at 433-443 K, this is due to the fact that when primary alcohol is treated with sulfuric acid, the alcohol undergoes intramolecular dehydration and forms alkenes. The reaction is:
CH3−CH2OHH2SO4443KCH2=CH2+H2O
Now, this ethene is oxidized to form formaldehyde. This takes place when the ethene is oxidized with ozone. When ethene is treated with ozone the double bond will be attacked and it will convert into 2 moles of formaldehyde. The reaction is given below:
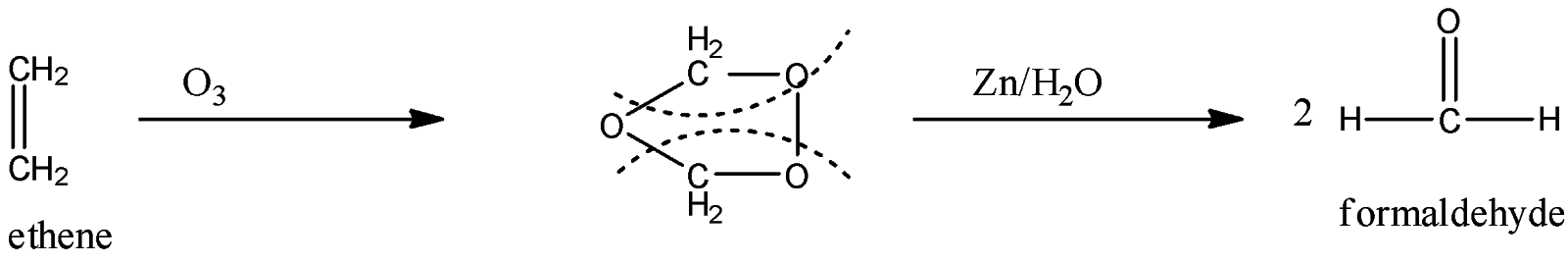
In the above reaction, the solvent must be used like carbon tetrachloride, chloroform, etc at low temperature for the reaction to proceed smoothly.
Note: If propene is used for ozonolysis, there will be the formation of two products i.e., ethanal and methanal. In the ozonolysis, the transition state formed is called Ozonide and it is highly unstable.
