Question
Question: How will you convert phenol to benzene?...
How will you convert phenol to benzene?
Solution
Benzene in the laboratory is prepared from the process which involves the reduction of Phenol. The method is commercial and so chosen as a suitable procedure to produce Benzene.
Complete answer:
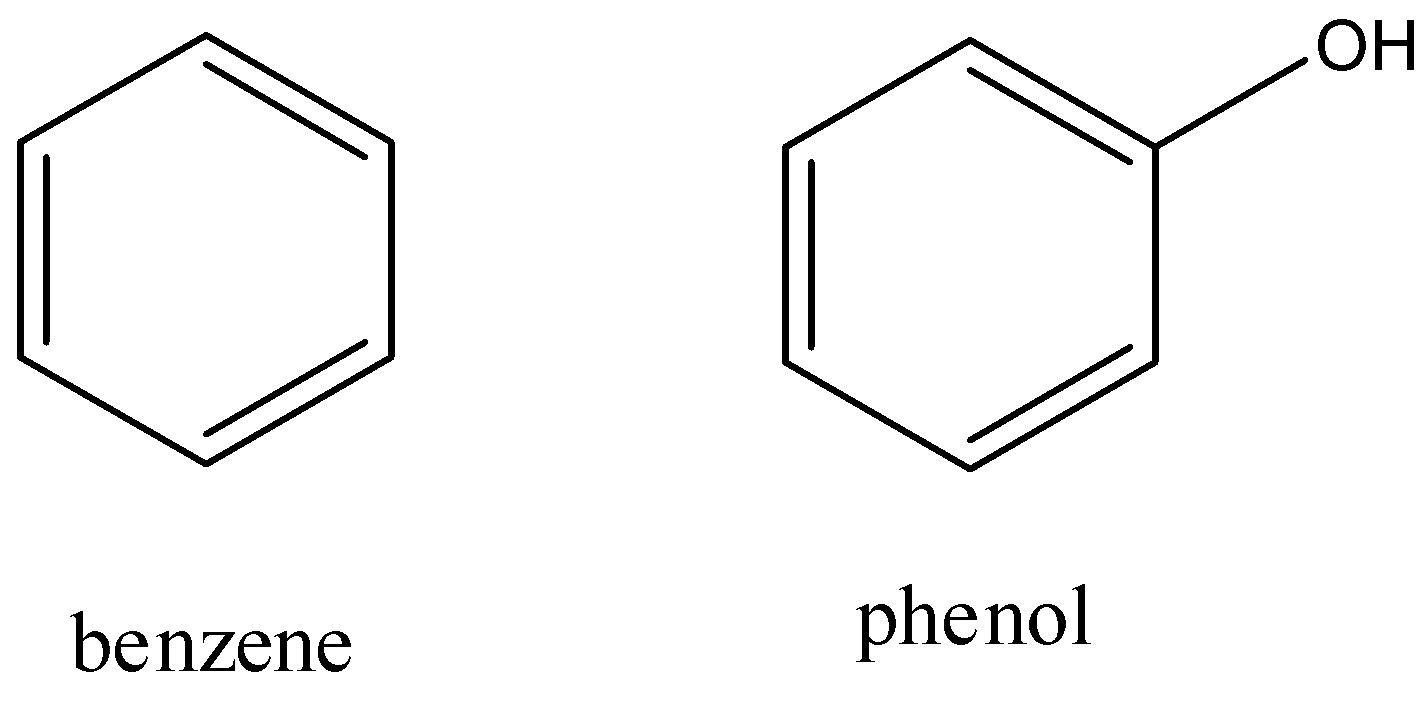
First, let us look at the structures of benzene and phenol. Benzene is a six membered ring with alternating double and single bonds; each carbon atom has one hydrogen atom attached to it. Phenol has a structure similar to benzene except one of the hydrogen atoms attached to carbon is replaced by a hydroxyl group. The skeleton structures are as follows:
Now, to form benzene from phenol, the commonly used industrial method is to reduce benzene using red hot zinc powder and distillation. The reaction is as follows:
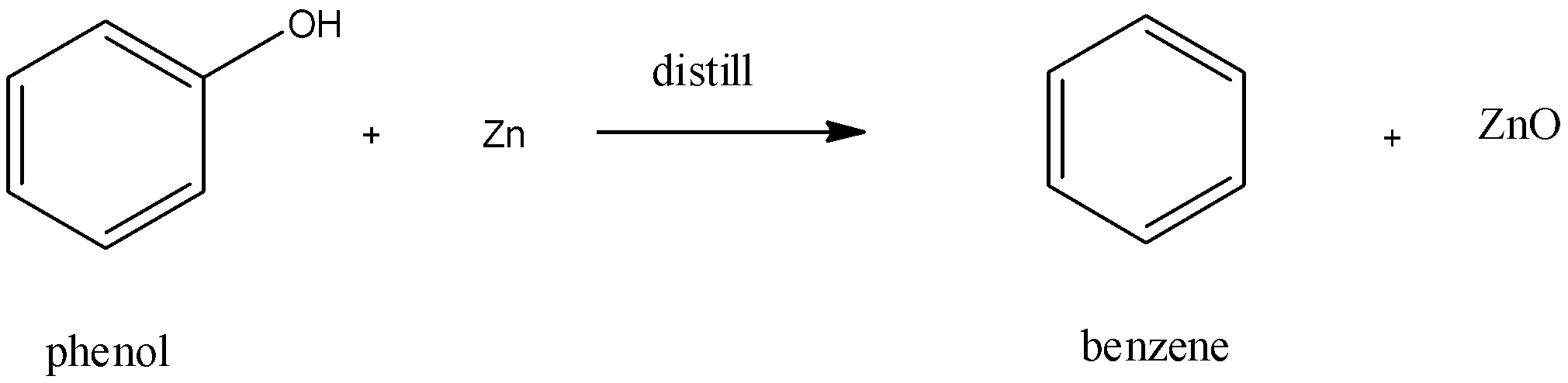
(−OH group of the benzene nucleus is replaced by −H in this reaction)
The above figure shows the conversion of phenol into benzene. Phenol gives out proton and forms phenoxy anion. This anion later gives out oxygen radical to form phenyl - free radical. On the other hand, zinc reacts with the oxygen radicals to form Zinc Oxide (ZnO). Finally, hydrogen free radical (formed from hydrogen ion) joins with phenyl - free radical to form Benzene.
Note:
The molecular formula of benzene is C6H6 this indicates that it is a highly unsaturated compound and is thus unstable. But a lot of stability is seen when benzene reacts with other substances; this happens due to a phenomenon called resonance. The resonance keeps the benzene ring stable. The formation of free radicals in this reaction will require a lot of energy, so the zinc has to be red hot so that the reaction can move forward.
