Question
Question: How will you convert ethyl chloride into (i) ethane (ii) n-butane?...
How will you convert ethyl chloride into (i) ethane (ii) n-butane?
Solution
Ethyl chloride and ethane have the same number of carbon atoms and only differ in one chloro substitution in place of hydrogen. n-butane contains four carbon atoms which is more than two carbon atoms of ethyl chloride, so some homologation reaction is needed.
Complete step by step answer: The given compounds belong to organic compounds. Ethyl chloride is an alkyl halide in which an ethyl group is attached to a chlorine atom. The parent hydrocarbon is ethane which contains the same number of carbon atoms as ethyl chloride. The chemical formula of ethyl chloride is C2H5Cl and ethane is C2H6.
The other molecule n-butane is a straight chain alkane compound with four carbon atoms. The chemical formula of n-butane is C4H10.
Let us determine the conversion of ethyl chloride into ethane and n-butane one by one. The ethyl chloride contains a good leaving group which is the chloride ion denoted as Cl−. So treatment of ethyl chloride with strong alcohol KOH leads to elimination of a molecule of HCl through E2 elimination mechanism and generates an alkene. The alkene product is ethene. The ethene upon reduction or hydrogenation with Raney Ni leads to formation of the desired compound ethane. The reaction is shown as
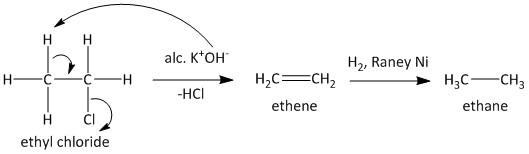
The second conversion from ethyl chloride to n-butane is achieved using Wurtz reaction. Wurtz reaction is widely used as a dimerization reaction for the formation of alkanes from corresponding alkyl halides. The alkyl halide is treated with metallic sodium to produce corresponding alkyl sodium which reacts with another molecule of alkyl halide to generate the dimer product. Thus ethyl chloride reacts with sodium and produces an ethyl sodium compound which in reaction with other ethyl chloride molecules leads to generation of n-butane. The reaction is shown as
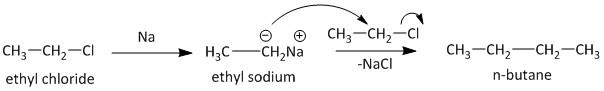
Note:
The alkyl halides on treatment with aqueous KOH, leads to formation of alcohols but with alcohol KOH generates alkene. The Wurtz reaction is limited to symmetrical alkanes. A side product alkene is formed during Wurtz reaction of bulky alkyl halides.
