Question
Question: How will you convert ethanol to propan-\(\mathbf{2}\)-ol?...
How will you convert ethanol to propan-2-ol?
Solution
For converting ethanol to propan 2-01, follow these steps:
-Converting ethanol to ethanol: By carrying out oxidation reaction, are can convert ethanol to ethanol. Ethanol contains alcohol and its oxidation is carried out in the presence of pyridinium chlorochromate (PCC). Once this oxidation is carried out, it cannot be oxidized further.
-Converting Ethanol to Propan-2-ol. A reagent called brignand when reacted with aldehyde, it gives 2∘ alcohol, which is our final product.
Complete step by step answer:
Firstly, we should have an idea about the ethanol, its characteristics, structure etc.
-Ethanol, also known as Ethyl Alcohol, a member of the alcohol family. Its chemical formula is C2H5OH.
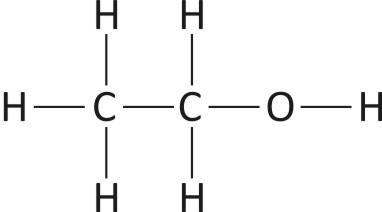
Fig: Structure of Ethanol
-In order to convert Ethanol to propan-2-ol, we have to first convert ethanol to ethanol.
For this we have to carry out the oxidation reaction of Ethanol.
-As we all know about the oxidation process, oxidation is the loss of electrons by an atom or the ion during the reaction. To carry out this oxidation reaction, we have to use a reagent called pyridinium chlorochromate (PCC). Then 1∘ alcohol present in ethanol is converted to aldehyde.
The oxidation of ethanol is carried out like this,CH3OH2OHC→PCCCH3CHO
-Here, we get the ethanol after carrying out oxidation reaction.But are need propan-2-al which contains three carbon containing alcohol.l So we will use Grignard reagent which will contain a methyl group along with the magnesium bromide and a methyl group will be attached to ethanol
(Ethanol)CH3CHO+(Grignand !!′!! s Reagent)CH3MgBr→(Propan-2-ol)(CH3)2CHOH
So,n by in this way, we can convert ethanol to propan-2-ol.
Additional information: Pure ethanol is a highly flammable, colourless liquid in appearance. It’s boiling point is 78.5∘C.
Ethanol is an important chemical used as a solvent in manufacturing of other chemicals.
Propan-2-ol is a colourless liquid, having odour like pungent alcohol. It’s density is 0.789 gm/cm3.
Propan-2-ol is widely used as a solvent, sterilizing agent and it is found in many skin lotions, mouthwashes and cleaning liquids.
Note:
It should be noted that proper reagents should be added to the compound to carry out correct reactions in order to get the desired product.
As we know our reagents and final products are highly flammable so every step should be followed with full concentration and proper care.
