Question
Question: How will you convert ethanoic acid into benzene?...
How will you convert ethanoic acid into benzene?
Solution
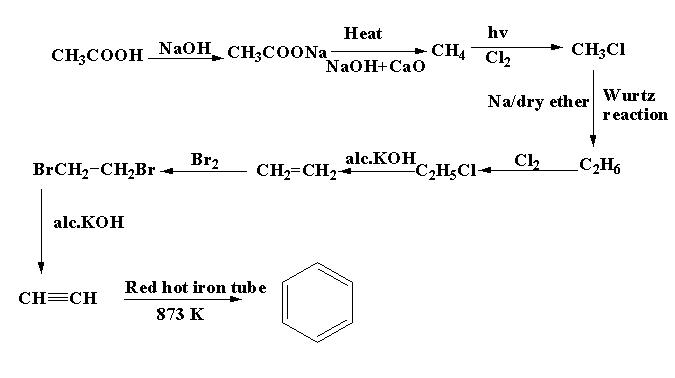
The ethanoic acid cannot be converted into benzene by one or two steps. Total nine steps are involved in the conversion of ethanoic acid to benzene. The main reactions involved are chlorination, bromination, Wurtz reaction.
Complete step by step answer:
For converting ethanoic acid CH3COOH to benzene C6H6 , a number of steps are involved. First the ethanoic acid is reacted with base sodium hydroxide NaOH, the product formed is sodium acetate CH3COONa. After that, the sodium acetate is heated with sodium hydroxide and calcium oxide, methane CH4 is formed as the main product and sodium carbonateNa2CO3 is formed as the by product. The methane is then reacted with chlorine in presence of light to form methyl chloride CH3Cl. The methyl chloride is then reacted with sodium metal in dry ether to form ethane C2H6. The reaction is known as Wurtz reaction. The ethane then undergo chlorination to form ethyl chloride C2H5Cl
. The methyl chloride is then reacted with alcoholic potassium hydroxide to form ethene CH2=CH2. The ethene then undergoes bromination to form 1,2-dibromoethane. BrCH2−CH2Br. The compound 1,2-dibromoethane is treated with alcoholic potassium hydroxide to form ethyne. Ethyne is when passed through a red hot iron tube at 873 K, benzene C6H6 is formed.
The reaction scheme for the preparation of benzene from ethanoic acid is shown below.
Note:
Make sure that the potassium hydroxide used in the reaction is alcoholic in nature, if aqueous potassium hydroxide is used instead of alcoholic potassium hydroxide then the product formed will be alcohol. The alcoholic potassium hydroxide is prepared by mixing potassium hydroxide solution with ethanol.
