Question
Question: How will you convert Benzene to Benzoic acid ?...
How will you convert Benzene to Benzoic acid ?
Solution
Hint: Benzoic acid means COOH group presence. Alkylation followed by further oxidation in the presence of oxidizing agent makes it easier for the conversion of benzene into Benzoic Acid.
Complete step by step answer:
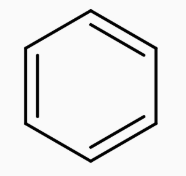
The figure represents the structure of benzene.Our First step is to convert benzene to Toluene. This can be achieved by Alkylation of benzene with CHCl3.Along with anhyd AlCl3,the whole process is called the Friedel Craft Alkylation Process.
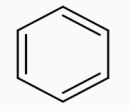
anhyd.AlCl3→CHCl3 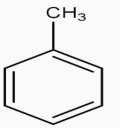
2) Next step is to convert This methyl group into COOH group by the help of strong oxidation agent KMnO4,the reaction takes place through oxidation.
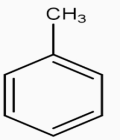 [oxidation !!]!! →KMnO4
[oxidation !!]!! →KMnO4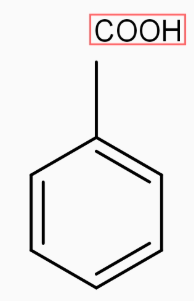 .
.
So, we finally get our final product as Benzoic Acid.
Additional Information: Benzene was first discovered by the English scientist Michael Faraday in 1825 in illuminating gas. In 1834 German Chemist Eilhardt Mitscherlich heated benzoic acid and produced benzene. Benzene is the natural constituent of crude oil and is also one of the most important elements of petrochemical. Due to its cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon.
Chemical Properties of benzene and benzoic acid:
- Benzene is immiscible in water but soluble in organic solvents.
- It is colourless liquid and has an aromatic odour.
- Benzene shows resonance.
- It has a density of 0.87gcm−3 .
- Benzoic acid is an aromatic carboxylic acid.
- Benzoic Acid has a colorless appearance in its solid state, which is of crystalline nature.
Note: We can also convert benzene into benzoic acid by the following steps also:
first convert benzene into benzaldehyde by treating benzene with CO and HCl in the presence of AlCl3,And formylation takes place to give benzaldehyde.
Second Step: We can now convert benzaldehyde into benzoic acid in the presence of oxidizing agents. So don't confuse yourself which process should be followed, Both the processes are fine.
