Question
Question: How will you convert benzene into N, N – dimethylaniline....
How will you convert benzene into N, N – dimethylaniline.
Solution
the benzene is nitrated followed by the reduction in presence of H2/Pd.the aniline formed further reacted in presence of chloroform CH3I such that each hydrogen of the aniline is replaced by methyl −CH3to produce the N, N – dimethylaniline.
Complete step by step answer:
The benzene is first converted into the nitrobenzene. To do so, the benzene is treated with the nitration mixture (H2SO4+HNO3) .the nitration is an electrophilic substitution reaction.
The mechanism of nitration of benzene.
Step 1) Nitric acid accepts the proton from the sulphuric acid and generates the nitronium ion.
Step 2) The nitronium ion acts as an electrophile and it attacks the benzene to form arenium ion.
Step 3) the arenium ion then loses its proton and forms nitrobenzene.
The nitrobenzene undergoes a selective chemical reduction in presence ofH2/Pd. This reduces the nitro group into the amino group and converts the nitrobenzene into the aniline. The reduction is a selective chemical reduction, in which the nitro group is selectively reduced to the aniline but the benzene ring is not reduced.
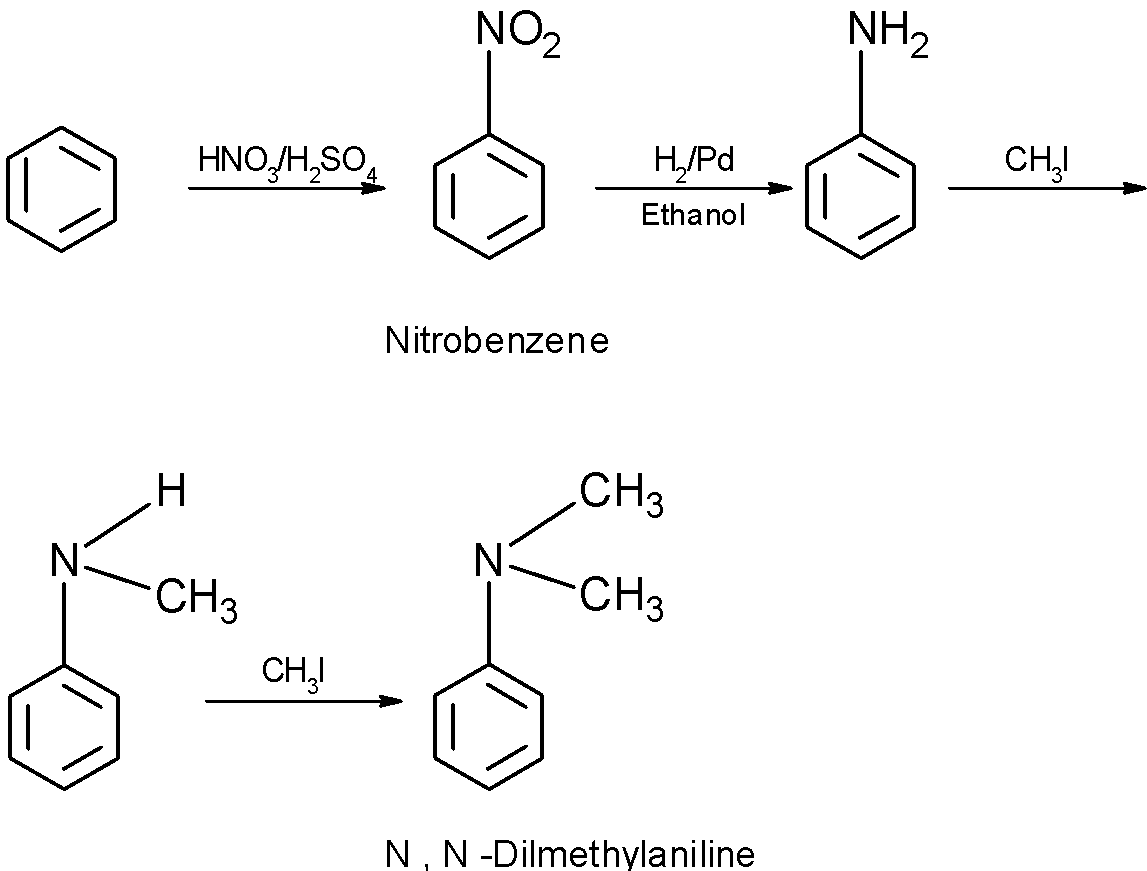
The aniline obtained then further undergoes the reaction. The N, N – dimethylaniline is the product of methylation of aniline. When aniline is treated with the methyl iodide CH3I , the N-methyl aniline is obtained, and further treatment of N-methyl aniline with the next molecule of CH3Iproduces N, N – dimethylaniline. The further methylation produces the trimethylamine. It is a quaternary ammonium salt.
Thus, through the above route, the benzene can be converted into the N, N – dimethylaniline.
Note: The nitrobenzene can be reduced through various routes. It reduces in concentrated HCl and generates the aniline, however when reduced in alkaline medium nitro benzene forms the intermediate compounds like nitrosobenzene and Phenyl hydroxylamine which further undergo bi-molecular condensation reaction.
