Question
Question: How will you convert benzaldehyde into following compounds? More than one step be required. (i) B...
How will you convert benzaldehyde into following compounds? More than one step be required.
(i) Benzophenone
(ii) Benzoic acid
Solution
Benzaldehyde is a compound having an aldehyde group attached to benzene. Benzophenone is a compound having two benzene groups connected with a carbonyl group. While benzoic acid is a compound having carboxyl groups attached to benzene. Benzene to benzoic acid is a simple step conversion while the other involves two to three steps.
Complete step by step answer:
(i) When aldehyde is oxidized, a carboxylic acid is obtained. Benzaldehyde is the simplest aromatic aldehyde. It has an almond-like odor. Benzoic acid is a colorless solid. The chemical formula of benzoic acid is C6H5COOH.
Thus benzaldehyde is converted to benzophenone in three steps:
Oxidation of benzaldehyde to benzoic acid.
Benzaldehyde is oxidized to benzoic acid in the presence of alkaline potassium permanganate. Since benzaldehyde possesses a benzene ring, it facilitates the electrophilic substitution reaction. The chemical equation for oxidation of benzaldehyde to benzoic acid is given below:
C6H5CHOKMnO4C6H5COOH
Benzaldehyde Benzoic acid
Conversion of benzoic acid to benzene.
Benzoic acid is heated with soda lime which is a mixture of NaOH and CaO, benzene is obtained. The reaction is given below:
C6H5COOHNaOHCaOC6H6
Conversion of benzene to benzophenone.
Benzene is reacted with benzoyl chloride, C6H5COCl in the presence of AlCl3, benzophenone, C6H5COC6H5 is obtained. The chemical equation is given below:
C6H6+C6H5COClAlCl3C6H5COC6H5
This reaction is called Friedel Crafts acylation.
Note:
Benzaldehyde can also be converted to benzoic acid by Cannizaro reaction. Cannizaro reaction is a type of disproportionation reaction. This involves disproportionation of an aldehyde which lacks α− hydrogen atom to salt of an acid and a primary alcohol. The reaction is given below:
2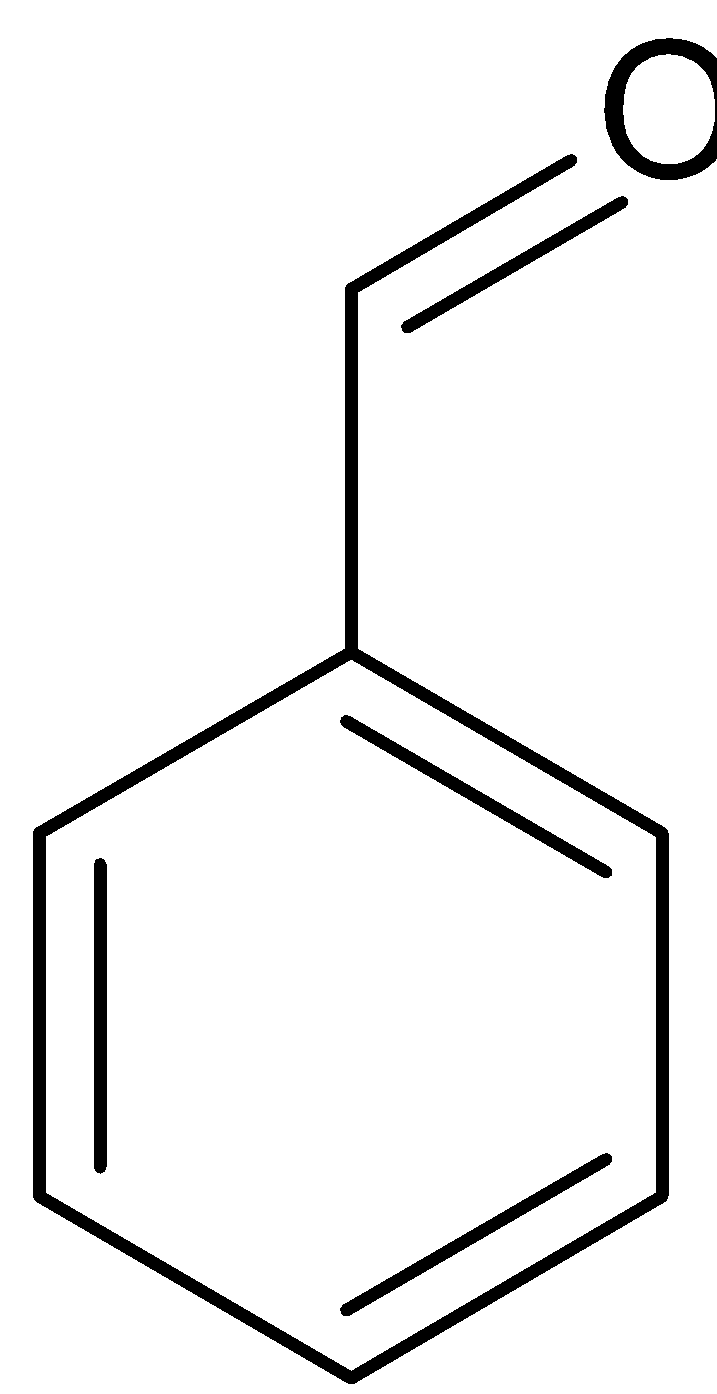 +NaOH→
+NaOH→ 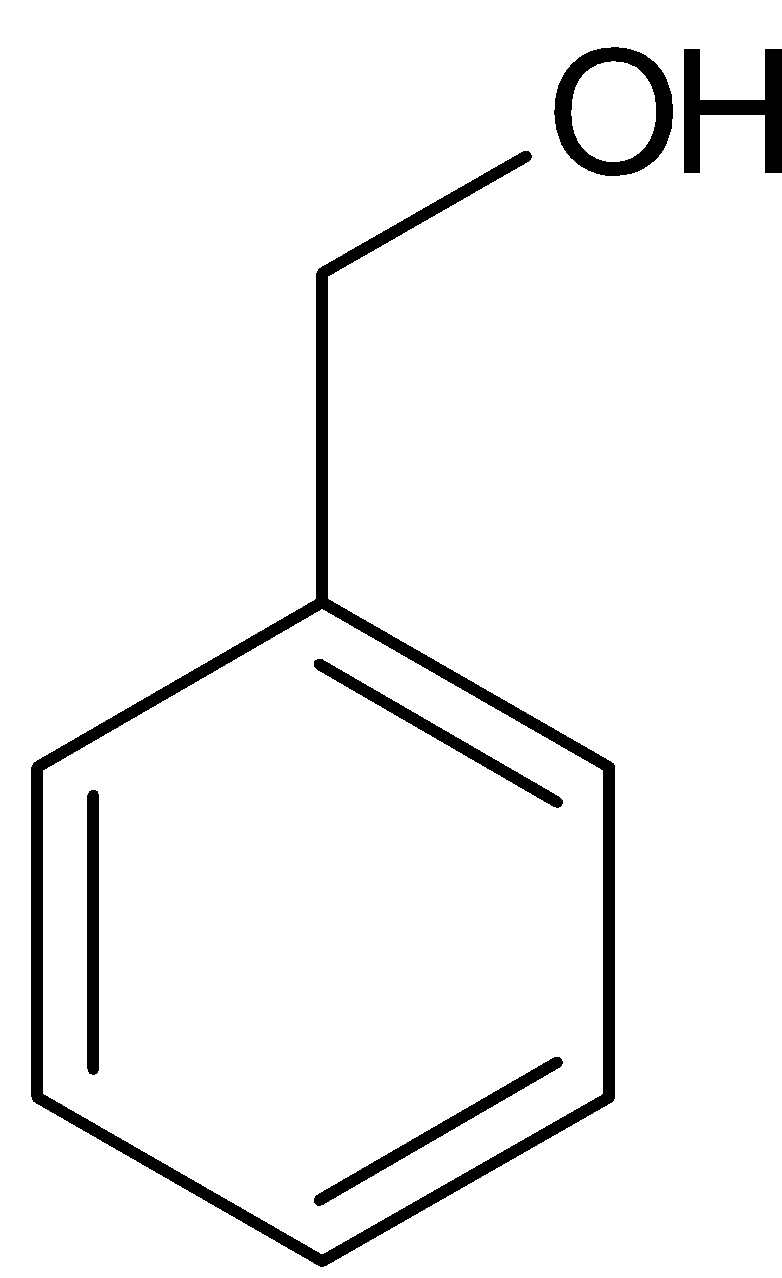 +
+ 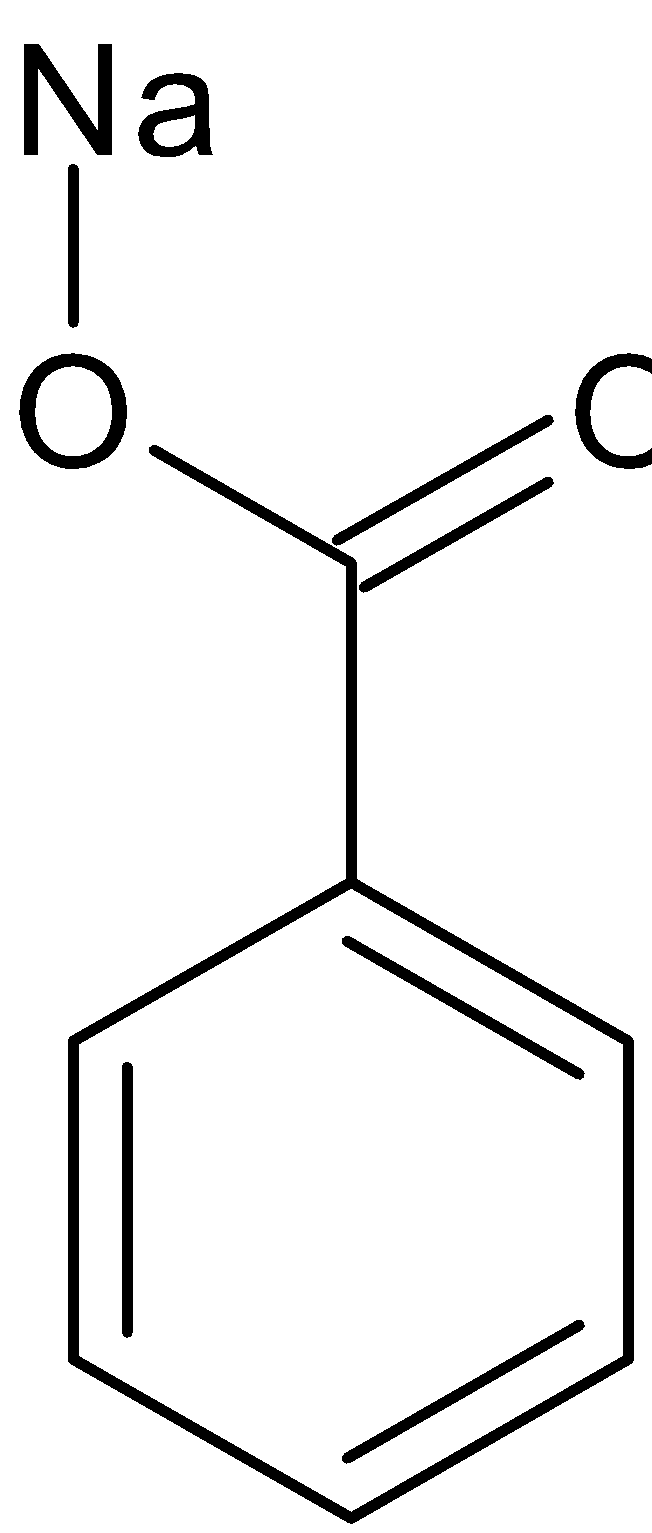 H+
H+ 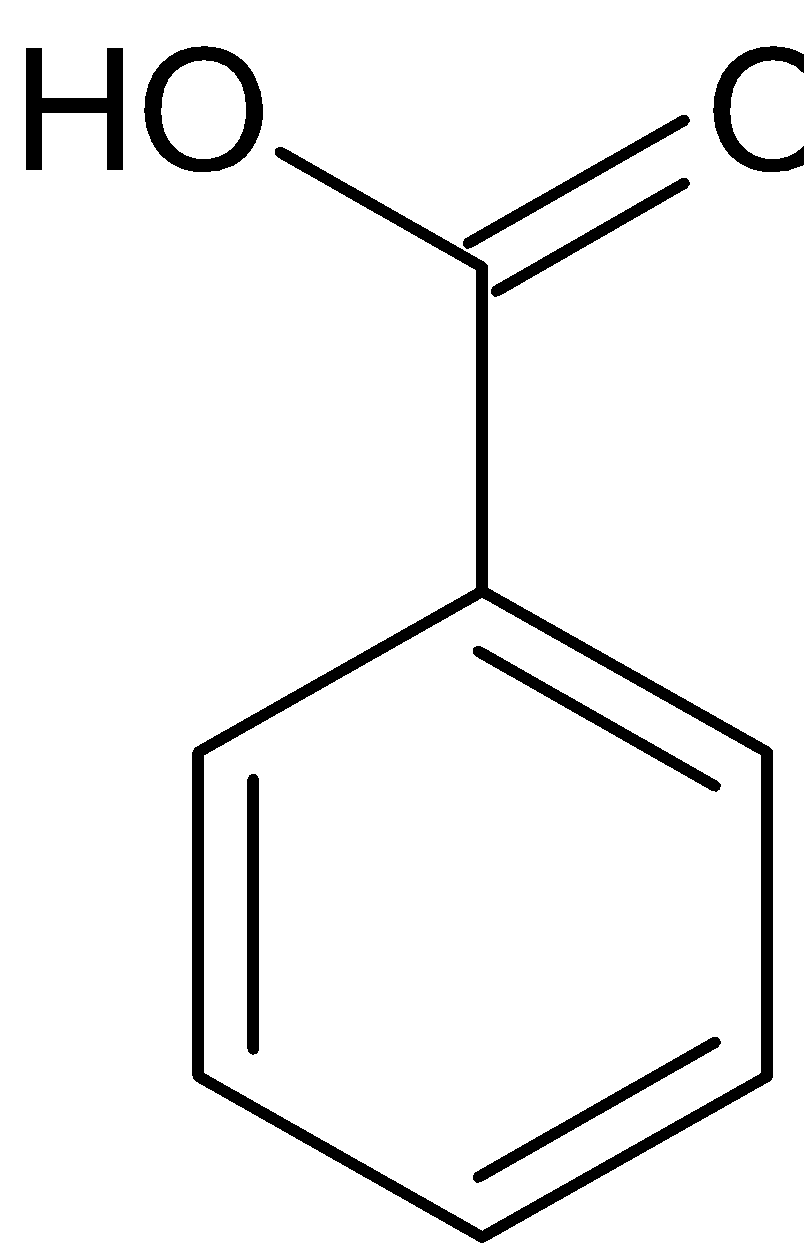
Benzaldehyde Benzyl alcohol Sodium benzoate
