Question
Question: How will you convert aniline to benzene?...
How will you convert aniline to benzene?
Solution
Aniline is the common name for compound in which - NH2 functional group is attached with benzene. Since - NH2 group is not a good leaving group so we convert it into a good leaving group by diazotizing it.
Complete step by step answer:
First we will convert the aniline to benzene diazonium chloride to make amine a better leaving group
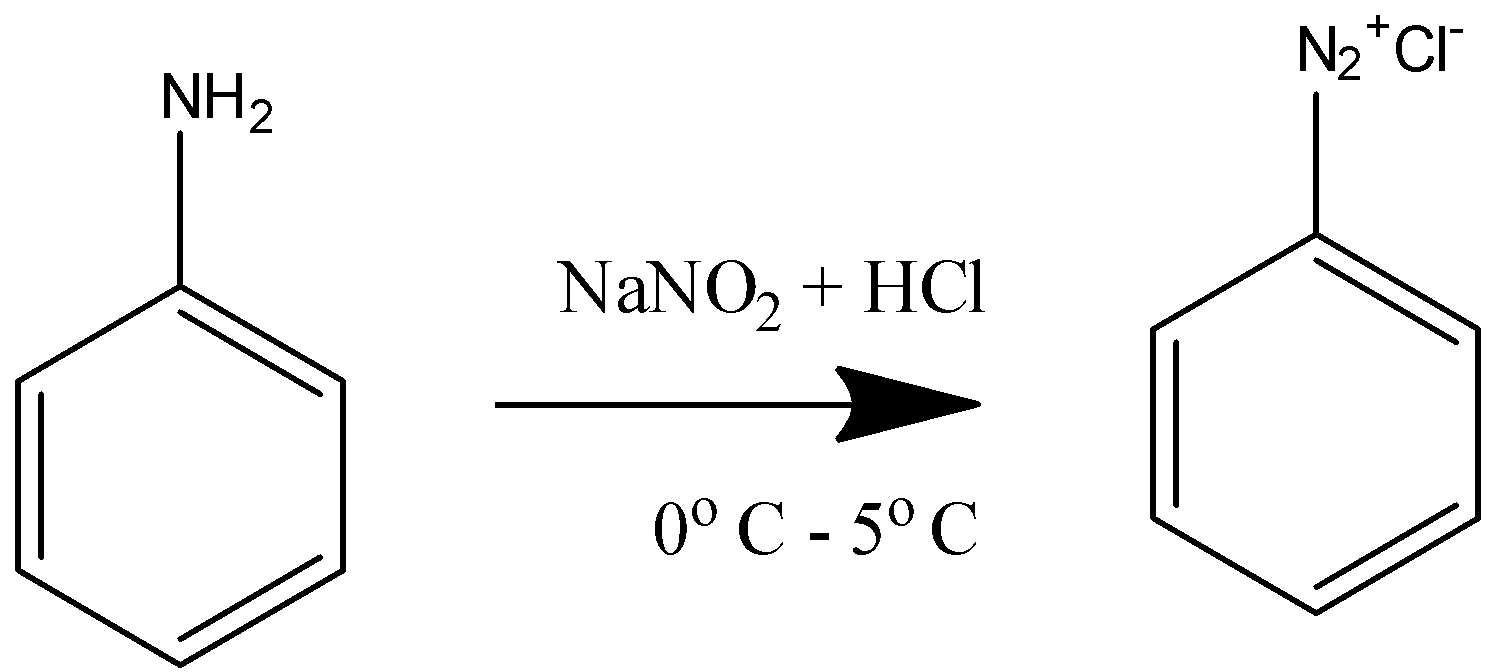
Aniline Benzene diazonium chloride
This is the diazotization of the amine group into diazonium salt with the help of NaNO2 + HCl at {\text{0^\circ C}}
This step is the most important step.
Now we will react this benzene diazonium chloride with hypophosphorous acid to convert it to benzene.
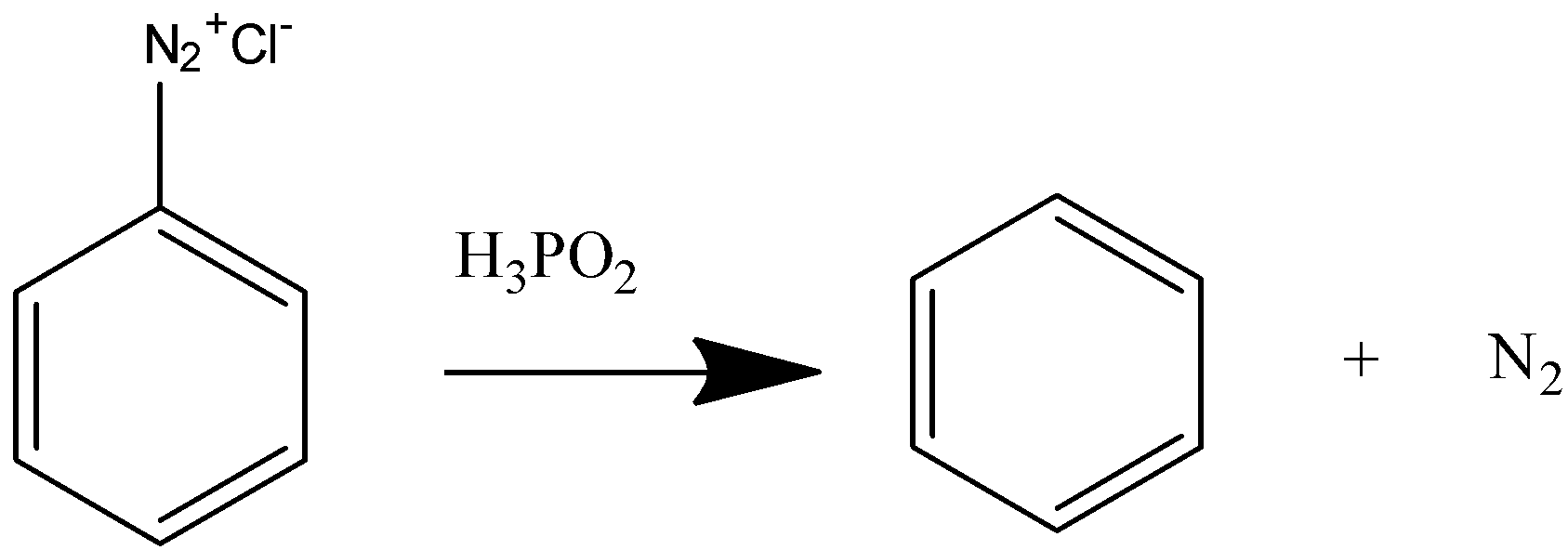
On reacting with hypophosphorous acid the diazonium salt releases nitrogen gas and benzene is formed.
In the above reaction the first step is very important as it increases the leaving capacity of nitrogen. The reagents NaNO2 + HCl gives HNO2 which converts the - NH2 group into oxime group - N = N - OH
This oxime is unstable and reacts with remaining HCl in the container to form N2 + Cl - which is called diazonium chloride. This diazonium can react with a variety of different reagents to form a large variety of different products from those compounds who have poor leaving groups. The poor leaving group converts to a very good leaving group like diazonium chloride and forms a product further.
Note: After diazotization the benzene diazonium chloride salt can be converted to fluoro-benzene by Schiemann’s reaction , chloro-benzene by Sandmeyer reaction , iodo-benzene by Gattermann reaction, benzene by hypo-phosphorus acid etc.
The mixture of NaNO2 + HCl should be freshly prepared so that only HNO2 is formed otherwise it will convert to HNO3 nitric acid which cannot be a nucleophile for this reaction.
