Question
Question: How will you convert- (a) Ethanoic acid into propanoic acid (b) Propanoic acid into ethanoic ac...
How will you convert-
(a) Ethanoic acid into propanoic acid
(b) Propanoic acid into ethanoic acid
Solution
Hint: We know that for the conversion of the given compounds we need to perform several reactions in order to get our desired products. While performing these chain reactions we need to remember some important reactions where we need to reduce or increase the number of carbon atoms.
Complete step by step solution:
(a) Ethanoic acid into propanoic acid – We know that carboxylic acids can be converted into alcohols by treating them with lithium aluminium hydride, so here we have treated ethanoic acid with LiAlH4 and got ethyl alcohol. When ethyl alcohol is treated with potassium iodide it produces ethyl iodide which further takes part in the reaction with potassium cyanide and produces ethyl cyanide. At last when ethyl cyanide reacts with H2O/H+ it gives propanoic acid.
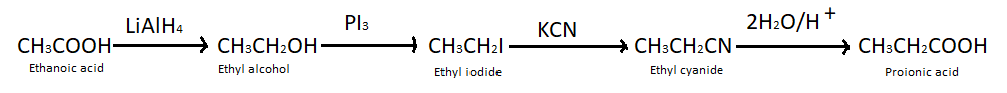
(b) Propanoic acid into ethanoic acid - To convert propanoic acid into ethanoic acid first of all we heat propanoic acid with ammonia which produces propenamide (an ammonium salt). Then we treat propenamide with bromine and aqueous or alcoholic KOH which gives ethylamine, so here one carbon atom is reduced from the compound. This reaction is also known as Hoffmann bromamide degradation reaction. We have studied that when nitrous acid reacts with amine it produces alcohol so we have treated ethyl amine with nitrous acid. After all of these reactions by performing oxidation of ethyl alcohol we got ethanol and again doing oxidation reaction we got our desired product that is ethanoic acid.
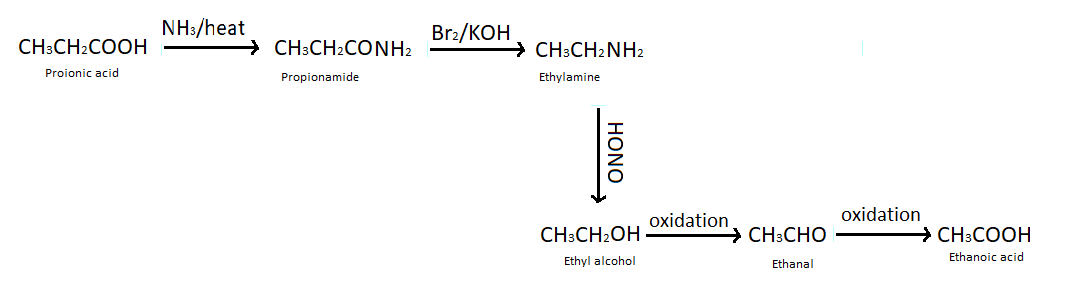
Note : Hence we have converted ethanoic acid into propanoic acids and propanoic acid into ethanoic acid with the help of reactions which is mentioned above in the solution.
