Question
Question: How \({{\rm{H}}_{\rm{2}}}{\rm{S}}\) is liberated in the laboratory? A.\({\rm{FeS}}{{\rm{O}}_{\rm{4...
How H2S is liberated in the laboratory?
A.FeSO4+H2SO4
B.FeS+dil.H2SO4
C.FeS+conc.H2SO4
D. ElementaryH2+elementary S
Solution
We know that, hydrogen sulphide (H2S) is a colorless gas which possesses the smell of rotten eggs. This gas is flammable and highly corrosive. Hydrogen sulphide is a very poisonous gas. It is mainly used in industries.
Complete step by step answer:
Let’s discuss the process of manufacturing hydrogen sulphide gas. The preparation of hydrogen sulphide in the laboratory is done by reacting iron sulphide with dilute sulphuric acid.
The reaction is as follows:
FeS(s)+dil.H2SO4(aq)→FeSO4(aq)+H2S(g)
The above reaction cannot be performed in presence of nitric acid and concentrated sulphuric acid because they oxidize hydrogen sulphide to sulphur.
Therefore, the correct answer is option B.
Additional Information:
Let’s discuss some important points regarding hydrogen sulphide. There is similarity in the structures of hydrogen sulphide and water.
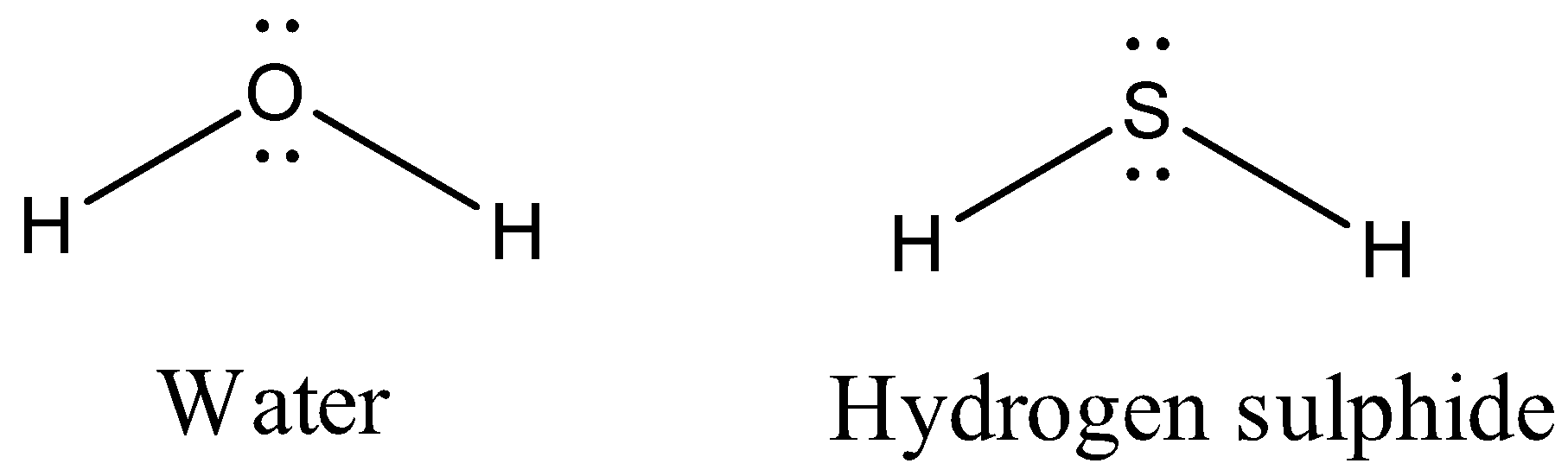
But remember that, the electronegativity of sulphur is less than oxygen. So, the polarity of hydrogen sulphide is less than water. This results in weaker intermolecular force in hydrogen sulphide than in water. Also the boiling and melting point is comparatively lower than water.
Let’s discuss chemical properties of hydrogen sulphide. At higher temperature, sulphur dioxide reacts with hydrogen sulphide in presence of catalyst to result in water and sulphur. This reaction is used to dispose of hydrogen sulphide.
Note:
The sulphur dioxide gas is flammable and highly explosive in nature. It is able to cause life threatening conditions if not handled with care. Moreover, this gas burns readily and releases toxic gases like sulphur dioxide. Also, fluid hydrogen sulphide can result in frostbite or blue skin.
