Question
Question: How many valence electrons are used to make sigma bonds in the molecule \[{C_3}{H_6}\]?...
How many valence electrons are used to make sigma bonds in the molecule C3H6?
Solution
Hint In order to find the number of valence electrons required to make a sigma bond in a molecule C3H6, we must first know what a valence electron is. Valence electrons are those electrons which are present in the outermost orbital or shell.
Complete step by step solution:
Let us first understand what a valence electron is. Valence electrons are those electrons which are present in the outermost orbitals or shells. Those electrons which are present in the inner shells are called the core electrons.
Let us move to the question. There will be formation of two compounds from the molecular formula C3H6. The two compounds which are formed will be cyclopropane and propene.in the molecular formula C3H6, two chemical elements will be present they are carbon and hydrogen. Carbon is having an atomic number of 6 and it will be having 4 valence electrons in it. As there are three carbon atoms in C3H6, then the total valence electrons present in carbon in C3H6 is 12. Hydrogen is having an atomic number of 1 and it will be having 1 valence electron. As there are six hydrogen atoms in C3H6, then the total valence electrons present in hydrogen in C3H6 is 6. Hence the total valence electron in C3H6 is 18.
The structure for cyclopropane is given below:
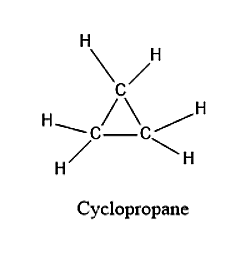
From the structure, we can see that there are 9 bonds present which are formed by mutual sharing of electrons. Hence there are 9 sigma bonds present.
The structure for Propene is given below:
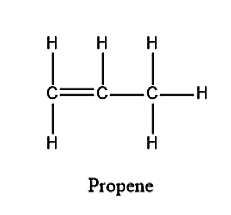
From the structure, we can see that there is a double bond in propene in which one is sigma bond and one is pi bond. Therefore, in propene there are 8 sigma bonds present.
Note: We have to remember certain points such as
- The valence electrons in transition metals exist in the inner shells.
- The valence electrons that are completely filled are chemically inert.
- The valence electrons can absorb or emit energy in the form of photons
