Question
Question: How many unpaired electrons are present in\(N{i^{2 + }}\)?...
How many unpaired electrons are present inNi2+?
Solution
Nickel is a silvery metal which has a slight golden tinge. It is hard and ductile. It is corrosion resistant and heat resistant. It is a good conductor of electricity. It is also a ferromagnetic metal. It loses two electrons to formNi2+.
Complete step by step answer:
Nickel is a metal which has atomic number of nickel is 28 and its electronic configuration is 1s22s22p63s23p63d84s2
This configuration is according to the decreasing order of energy of orbitals. We can represent the configuration of nickel as-
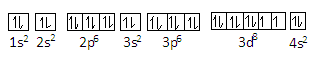
In this configuration nickel has 13 paired electron pairs and two unpaired electrons (in the 3d orbital).
Nickel due to its heat resistance property is used to make alloys of great strength as well as heat, corrosion and oxidation resistant.
Now Nickel donates two electrons to form Ni2+ ion. The reaction is given as-
Ni→Ni2++2e−
This means now the total number of electrons in the ion is 26 as the element loses the two electrons of 4s orbital so the electronic configuration of Ni2+ion is written as-
1s22s22p63s23p63d8
We can represent the configuration of Ni2+as-
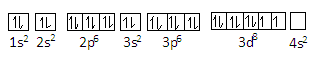
In this configuration, there are still 2 unpaired electrons present in the 3d orbital.
The correct answer is 2 unpaired electrons.
Note:
Nickel is used as-
-It is used in batteries
-It is used to plate other metals to protect them from corrosion.
-It is used to make alloys.
-It is used in toasters and electric ovens.
-It is used as a catalyst in many chemical reactions.
