Question
Question: How many unpaired electrons are in an atom of Co in its ground state? A. \( 7 \) B. \( 1 \) ...
How many unpaired electrons are in an atom of Co in its ground state?
A. 7
B. 1
C. 3
D. 2
Solution
In atomic or molecular orbitals, the distribution of electrons of an atom or molecule is the electron configuration. 27 is the atomic number of cobalt. Cobalt has the chemical symbol Co and belongs to the d-block of the periodic table. Cobalt in the periodic table is present in group 9 and period 4.
Complete Step By Step Answer:
Cobalt, a d-block element with symbol Co, has atomic number 27. The electronic configuration of cobalt is [Ar]3d74s2 . Electrons present in the outermost electronic configuration of an atom are the valence electrons.
9 is the valence electron in the cobalt atom. 7 electrons are present in the 3d orbital and 2 electrons in 4s orbital of the cobalt atom. The ground state molecular orbital structure of the cobalt atom will be:
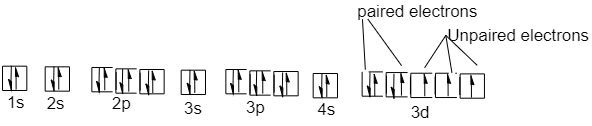
Electron filing takes place from lowest energy orbital to high energy orbital. In the d-orbital out of the 5 subshells, 2 subshells are totally occupied with two electrons of opposite spin, whereas the rest 3 subshells have only one electron each.
So cobalt has a total of 3 unpaired electrons present in its ground state.
Note:
Filling up of electrons in the orbitals of an atom is based on rules of the Aufbau principle, Pauli’s exclusion, and Hund’s rule. According to the Aufbau principle, the lower energy orbitals are filled first.
According to Pauli’s exclusion principle, only two electrons with opposite spins are allowed in an orbital, and Hund’s rule states that each orbital should be singly occupied in a sublevel before it is doubly occupied.
