Question
Question: How many types of ions are formed by heterolytic fission and what are their names?...
How many types of ions are formed by heterolytic fission and what are their names?
Solution
The majority of chemical reactions involve the breaking and creation of new chemical bonds. Chemical bonds, on the other hand, may be disrupted in a variety of ways. In addition, the way a chemical bond breaks is important in determining the overall result of a chemical process. Link fission refers to the breaking of a chemical link (typically a covalent bond). Homolytic and heterolytic fission are the two main forms of bond fission.
Complete answer:
Homolytic fission (also known as hemolysis) is a form of bond fission that includes the dissociation of a particular molecule in which each of the original pieces of the molecule retains one electron. As a result, when a neutrally charged molecule undergoes homolytic fission, the result is two free radicals (since each of the chemical species retains one electron from the bond pair).
Heterolytic fission, also known as heterolysis, is a kind of bond fission in which a covalent link between two chemical species is broken unevenly, with one of the chemical species retaining the bond pair of electrons (while the other species does not retain any of the electrons from the bond pair). One of the products of heterolytic fission of a neutrally charged molecule will have a positive charge, while the other will have a negative charge.
The chemical species that did not retain any of the bound electrons after the bond fission is known as the cation, which is the positively charged result of the heterolytic fission of a neutral molecule. The negatively charged heterolysis result (also known as the anion) on the other hand, is the chemical species that maintains both bound electrons following the bond fission process.
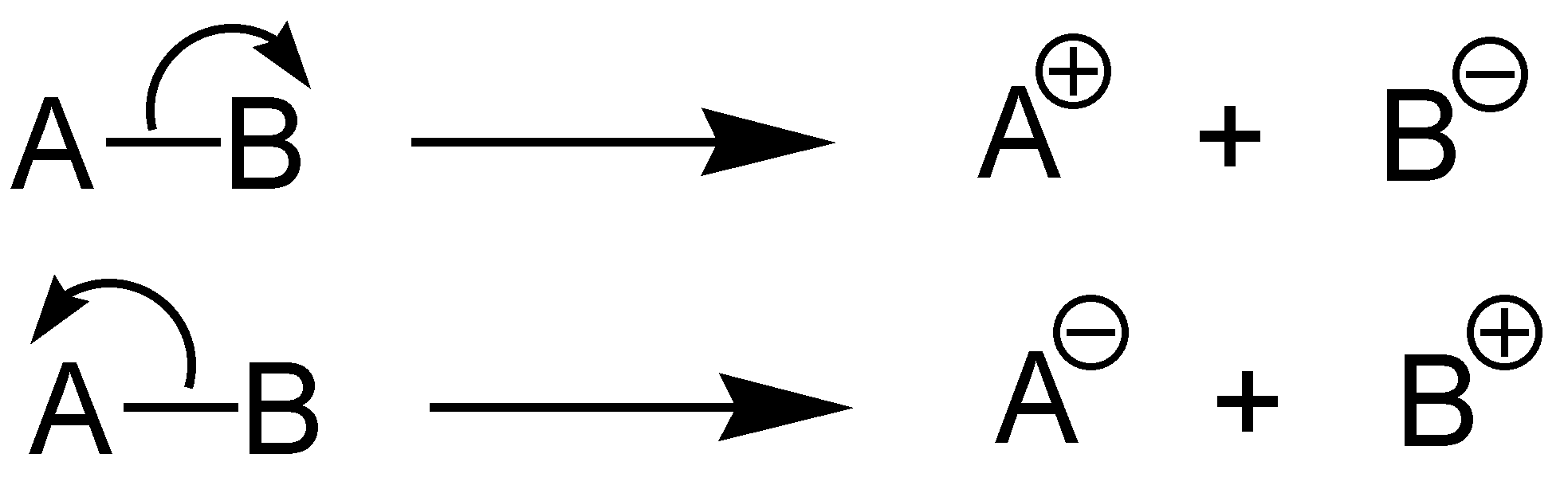
The term 'heterolysis' has Greek origins and approximately translates to 'unequal breaking.' Homolytic cleavage is another name for it. Below is a diagram depicting the two ways in which a molecule AB might undergo heterolytic fission. In the first case, B retains the bond pair of electrons, making it an anion and A a cation. A keeps the bond pair and becomes the anion in the second situation, while B becomes the cation.
Note:
It's also worth noting that when a covalent bond undergoes heterolytic fission, the bound species with the highest electronegativity generally keeps the bond pair of electrons and receives a negative charge. The more electropositive species, on the other hand, normally does not retain any electrons and acquires a positive charge.
