Question
Question: How many three member ring(s) is/are formed in\[Cr{O_5}\]?...
How many three member ring(s) is/are formed inCrO5?
Solution
Hint : We must remember that the member rings is the term that means the cyclic structure that is formed by a group of atoms and the bonds between them.
Complete step by step solution :
Let's first begin with explaining more about the member ring. Member rings are a vague term that we use while talking about the cyclic structure that is formed by a group of atoms and the bonds between them.
If a structure has no rings present in it then we call it an acyclic or open-chain compound. If the ring is a simple one, then we call it a monocycle and if it's relatively complex then we refer to it as a polycycle.
Another classification is the homocyclic and heterocyclic ring classification. A ring is called homocyclic if all the atoms are of the same element. A special class of homocyclic compounds are the carbocycle where all the elements are carbon. A heterocyclic has different elements in its ring.
In CrO5the Cr atom is having six valence electrons for bond formation and hence, forms the structure that is shown below. CrO5has five oxygen (O) atoms. Four oxygen atoms are involved in peroxide bonds. So CrO5have two linkages.
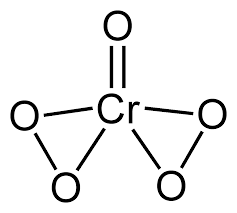
Looking at this structure we can see two distinct rings which are three membered.
So, the CrO5is having 2 three member rings.
Note : We must remember that when we calculate the oxidation state of a central atom. The calculated oxidation state of the central atom is greater than the maximum oxidation state of that atom. Then we can confirm there’s peroxide linkages present.
