Question
Question: How many \[{\text{s}}{{\text{p}}^{\text{2}}}\] hybridised carbon atoms are present in benzaldehyde?...
How many sp2 hybridised carbon atoms are present in benzaldehyde?
Solution
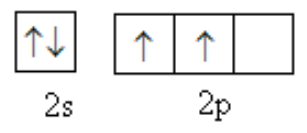
To determine the answer to this question we should the structure of benzaldehyde and the formation of sp2 hybridised atom. The sp2 hybridised orbital is formed by one s and two p-orbitals. Carbon atoms have one s and three p-orbital in the valence shell.
Complete step by step answer:
The structure of the benzaldehyde is as follows:
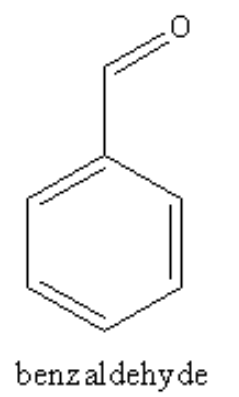
The valence shell of carbon is as follows:
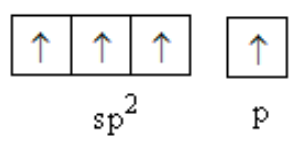
One s and two p-orbitals combine to form sp2 hybridised orbital of carbon.
Four un-hybridised p-orbital form pi bonds. Hybridised orbitals form sigma bonds only. So, we can say from the structure of the benzaldehyde that each carbon of the ring is forming three sigma bond and one pi bond so, each carbon of the ring is sp2 hybridized. The carbon of aldehyde is also forming three sigma and one pi bond so, the carbon of aldehyde is also sp2 hybridized.
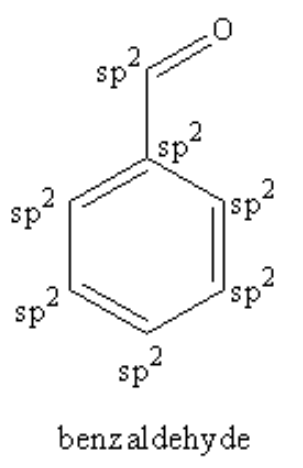
Therefore, 7 sp2 hybridised carbon atoms are present in benzaldehyde.
Note: The number of hybridized orbitals is equal to the number of sigma bonds. Hybridized orbitals have the same energy. The hybridization tells the number of hybrid orbitals. sp2 hybridization means three (one s and two p-orbital) hybrid orbitals or three sigma bonds. Each carbon of benzene ring issp2 hybridised. We can also determine the hybridization of an atom by counting the total number of sigma electron pairs around the atom. The oxygen atom has one sigma bond and two lone pairs, so a total of three electron pairs. Oxygen atom is also sp2 hybridised.in benzaldehyde a total of eight sp2 hybridised atoms are present.
