Question
Question: How many structural isomers with the molecular formula \({C_4}{H_{10}}O\) give infrared absorptions ...
How many structural isomers with the molecular formula C4H10O give infrared absorptions both at approximately 1200 cm−1 and at approximately 3400 cm−1?
a.) 2
b.) 4
c.) 6
d.) 7
Solution
Hint: The hydroxyl functional group (-OH) has a broad region of spectrum, generally 2500 to 4000 cm−1. The region below 1200 cm−1 is known as the fingerprint region. It accounts for the C-C, C-O and C-N vibrations. The region above 1200 cm−1 is known as the group frequency region.
Complete step by step answer:
The region of the infrared spectrum from 1200 to 700 cm−1 is called the fingerprint region. Many different vibrations, including C-O, C-C and C-N single bond stretches, C-H bending vibrations, and some bands due to benzene rings are found in this region. This region is usually the most confusing and complex region to interpret. However, the utility of the fingerprint region is that the many bands there provide a fingerprint for a molecule.
Many functional groups absorb infrared radiation at about the same wavenumber, regardless of the structure of the rest of the molecule. For example, C-H stretching vibrations usually appear between 3200 and 2800 cm−1 and hydroxyl(-OH) stretching vibrations usually appear between 2500 and 4000 cm−1. This makes these bands diagnostic markers for the presence of a functional group in a sample. These types of infrared bands are called group frequencies because they tell us about the presence or absence of specific functional groups in a sample.
Now, in the given question, as one IR absorption occurs at 3400 cm−1, then one -OH group is bound to be present in the structure. Also, as only one oxygen is present in the molecular formula of the compound, the other absorption at 1200 cm−1 is due to the C-C bond.
Hence, the total structures of C4H10O having hydroxyl group are 4
n-Butanol 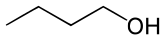
Sec-Butanol 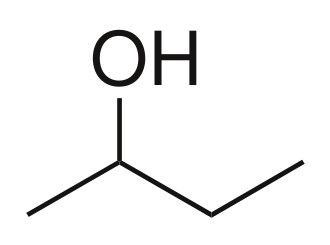
Tert-Butanol 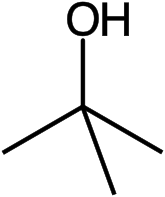
Iso-butanol
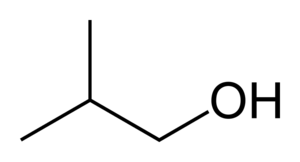
Hence, the correct answer is (B) 4.
Note: A student might confuse the absorption at 1200 cm−1 to be due to C-O bond which is not the case. Due to the presence of a single oxygen, then the absorption at 3400 cm−1 wouldn’t exist. When intermolecular hydrogen bonding occurs in the functional alcohol group, the IR frequency is accompanied by a shift to lower frequency.
