Question
Question: How many structural isomers do the molecules below have? The molecule below counts as one isomer. ...
How many structural isomers do the molecules below have? The molecule below counts as one isomer.
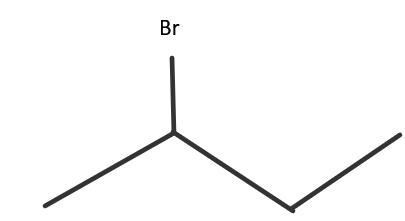
Solution
First try to figure out the IUPAC name of the given molecule. After that, try to draw the isomers by attaching the halide atom (bromine in this case) to different locations of the carbon chain. You should also try to relocate some carbon atoms to see whether they can become an isomer. Finally, write the IUPAC name of the isomers.
Complete step by step solution:
The molecular formula of the above molecule is C4H9Br and it is called 2− Bromobutane. Since, there is no specified stereochemistry of the molecule, there would likely be four constitutional isomers, including the one given in the question which are as follows:
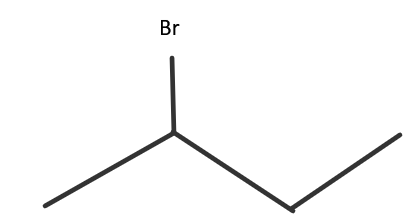
IUPAC name → 2− Bromobutane
This is the one given in the question. Here, the halide part is present at the second carbon atom of the chain. It is a 2∘ haloalkane. It is also called sec-butyl bromide.
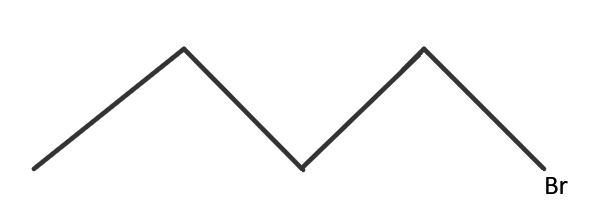
IUPAC name → 1− Bromobutane
This isomer is achieved when the bromine atom (halide) is present at the end of the carbon chain. It is a 1∘ haloalkane. It is also called Butyl bromide.
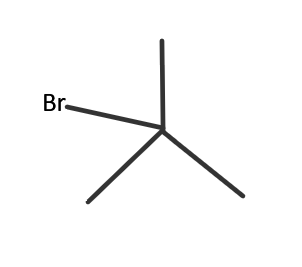
IUPAC name → 2− Bromo −2− methyl butane
This isomer is achieved when one carbon atom or one methyl group is present at the same carbon atom to which bromine atom (halide) is attached to. It is a 3∘ haloalkane. It is also called tert-Butyl bromide.
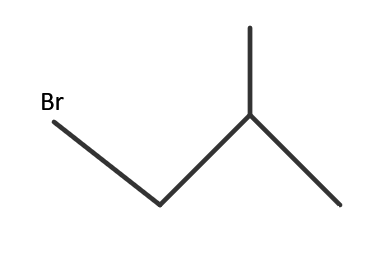
IUPAC name → 1− bromo −2− methylpropane
This isomer is achieved when the halide atom is present at the end of the carbon chain and one methyl group present at that carbon atom which is next to the terminal carbon atom. It is a 2∘ haloalkane. It is also called iso-butyl bromide.
Note:
While drawing the isomers, keep in mind that you might be drawing the same isomer, but just a different representation. For example, if you reverse the first isomer, the representation changes, however, it is still the same. Also note that the chemical formula of an isomer remains the same, therefore, check whether the isomers that you made have the same chemical formula or not.
