Question
Question: How many structural isomers are possible for a compound with molecular formula \( {{\text{C}}_{{\tex...
How many structural isomers are possible for a compound with molecular formula C3H7Cl ?
Solution
The chemical compounds which have the same molecular formula but differ in the arrangement of atoms in their structure are called structural isomers.
Complete Step By Step Answer:
Isomers are molecules having identical molecular but different structural formulae. They are classified as constitutional isomerism and stereoisomerism. Constitutional isomerism is exhibited by compounds which have the same molecular formula but differ in the arrangement of atoms whereas stereoisomers have the different arrangement of atoms in space. In other words, the stereoisomers differ in their spatial arrangement of atoms. This difference can lead to the change in physical and chemical properties.
Structural isomers are further classified into following types:
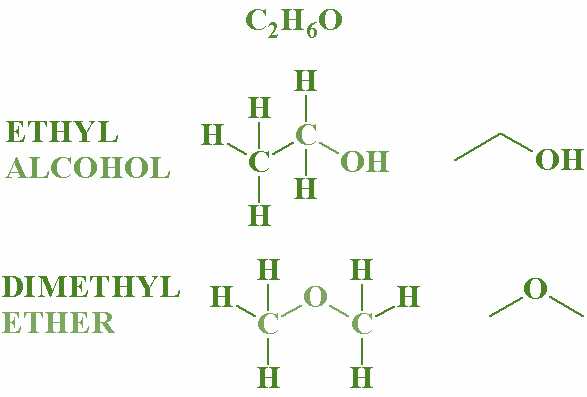
Functional isomers: Where the functional group of the compound differs.
Example- Alcohol and ether
Positional isomers: The position of atoms or functional groups gets different.
Example- Alcohols
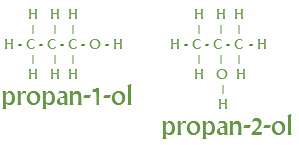
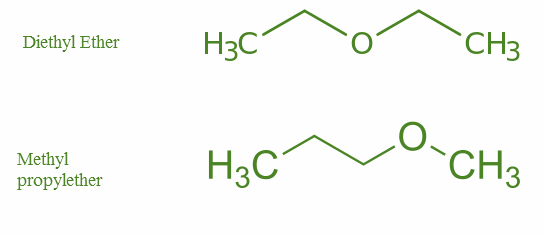
Metamerism: Where the number of atoms in the side chain of the functional group differs.
Example- Ethers
Chain isomerism: Differs in the carbon skeleton
Example-
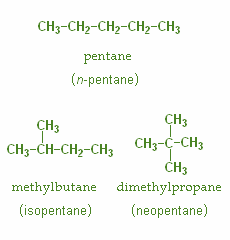
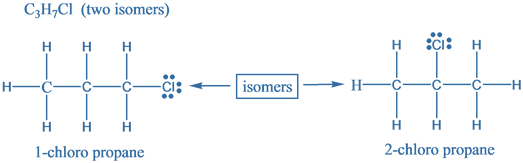
The given molecular formula is C3H7Cl. Structural isomers are molecules which differ in the arrangement of atoms but have the same molecular formula. These are also known as Constitutional isomers. Following are the structural isomers for the given molecular formula is C3H7Cl .
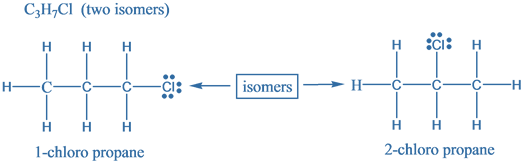
Hence, there are two structural isomers for a compound with molecular formula C3H7Cl .
So, the correct option is A.
Note:
The structural isomers can be distinguished by NMR spectroscopy with ease. Due to the different physical and chemical properties these isomers can also be separated easily. The change in name of compounds either in the numbering or functional group reflect structural isomerism.
