Question
Question: How many structural and geometrical isomers are possible for dimethyl cyclohexane? A.3,6 B.4,6 ...
How many structural and geometrical isomers are possible for dimethyl cyclohexane?
A.3,6
B.4,6
C.6,4
D.3, 3
Solution
We know that isomerism is the phenomenon in which the compounds have the same chemical formula but their structure is different. There are two types of isomerism that is structural isomerism and stereoisomerism.
Complete step by step answer:
Let’s first discuss structural isomerism in detail. Structural isomerism is shown by the compounds having the same molecular formulae differing in the arrangement of the atoms. There are four possible geometrical isomers for dimethyl cyclohexane.
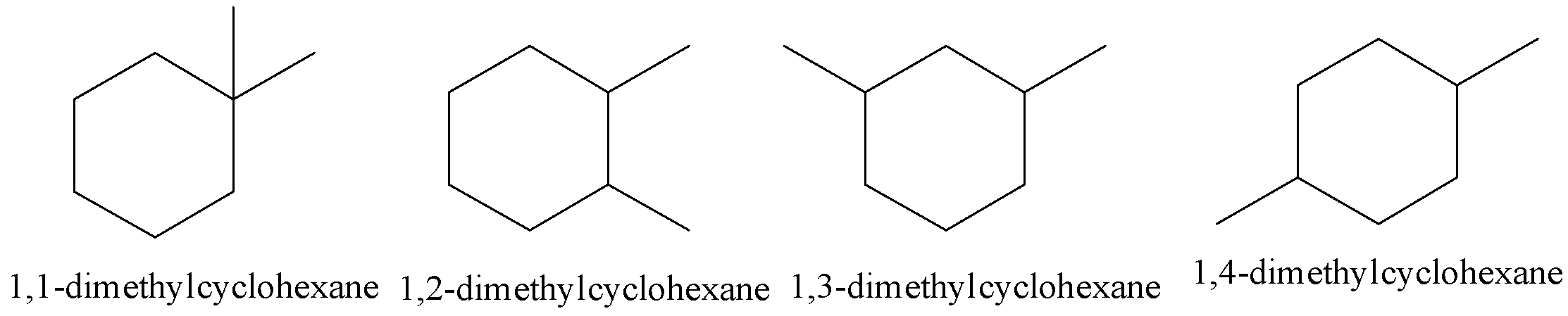
All the above structural isomers possess the same chemical formula but their structures are different.
Let’s discuss geometrical isomerism in detail. In geometrical isomerism, two types of isomers namely cis and trans present. Geometrical isomerism is a type of stereoisomerism.
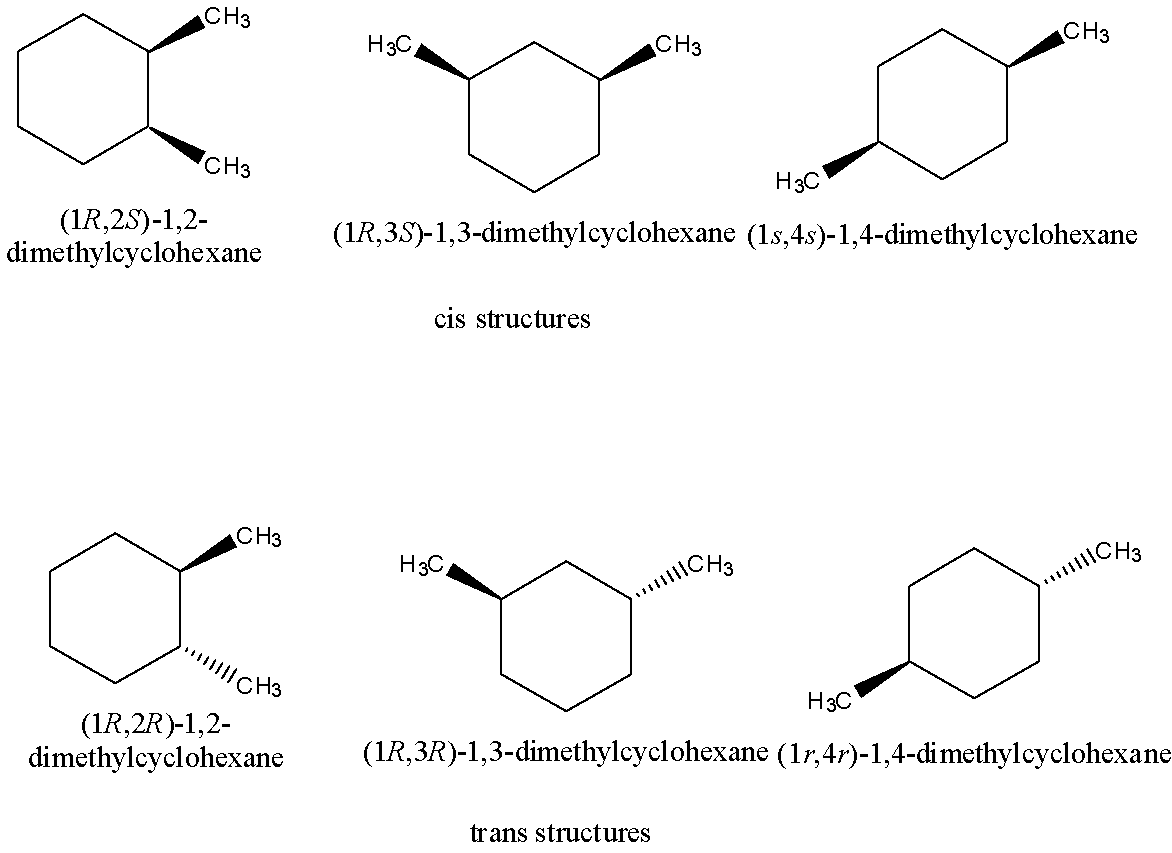
Out of six geometric isomers, three isomers are cis structures and three are trans structures. In geometrical isomers for dimethyl cyclohexane, cis structures are those structures possessing the two methyl groups on the same side of the benzene ring whereas the trans structures are those structures possessing the two methyl groups on the opposite side of the benzene ring.
Therefore, four structural isomers and six geometric isomers are possible for dimethyl cyclohexane.
So, the correct answer is Option B.
Additional Information:
The compounds having the same molecular as well as same structural formulae but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon is termed as stereoisomerism. There are six geometrical isomers possible for dimethyl cyclohexane.
Note: It is to be noted that structural isomerism is of different types depending upon the nature of the compounds involved. These are chain isomerism, position isomerism, functional isomerism, metamerism, tautomerism and ring chain isomerism.
