Question
Question: How many stereoisomers of \( \text{2 - chloro - 3 - methylbutane} \) exist?...
How many stereoisomers of 2 - chloro - 3 - methylbutane exist?
Solution
To know the existence of stereoisomers of 2 - chloro - 3 - methylbutane, we should go through the configuration of 2 - chloro - 3 - methylbutane. And then on the basis of configuration, we can conclude the stereoisomers.
Complete step by step solution:
Simply put, there are two configurations per stereo-centre on a compound with all sp3carbons;RorS . So, we rack up stereoisomers according to 2⋅2⋅2⋅[...]⋅2=2n where n is the number of stereo-centres.
Also, although meso isomers could reduce the number of stereoisomers, there are none here because clearly, Cl=CH3 , i.e. there is no plane of symmetry. Therefore, we can ignore meso isomers.
So, there are a total 22 or 4 stereoisomers of 2 - chloro - 3 - methylbutane exist in nature.
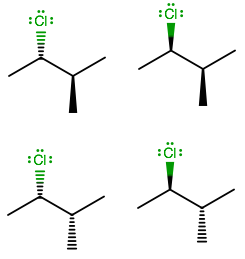
Note:
Stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers. Enantiomers are mirror images, like one’s hands, and diastereomers.
