Question
Question: How many species out of the following are aromatic? 
Solution
Aromatic compounds are considered as the compound which contains a conjugated planar ring system with delocalized pi-electrons cloud located at alternate double bond and single bond. For a compound to be aromatic it should follow Huckel’s rule which says that the compound should satisfy 4n+2π electrons.
Complete answer:
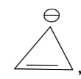
In the above given compound, 1 π bond is present which contains 2 electrons. Due to negative charge 2 electrons are present in the same plane. So, the total number of electrons is 4. This compound does not follow the 4n+2πrule. Therefore, it is antiaromatic.
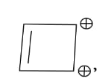
In the above given compound, 1 π bond is present which contains 2 electrons. It is an aromatic compound as it is cyclic, and planar. Due to positive charge all the carbon atoms become sp2 hybridized and it follows Huckel’s rule.
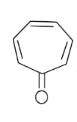
In the above given compound, 3 π bond is present which contains 6 electrons. The oxygen atom is not present in the plane, therefore its electrons are not counted. This compound is aromatic as it follows the 4n+2πrule.
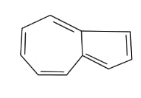
In the above given compound, 3 π bond is present which contains 6 electrons. The other cycle is not present in the plane, so its electrons are not counted. This compound is aromatic as it follows the 4n+2πrule.
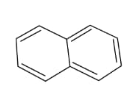
In the above compound, total 5 π bonds are present which contains 10 electrons. Therefore, this compound is aromatic as it follows the 4n+2πrule.
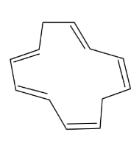
In the above compound, total 5 π bonds are present which contains 10 electrons but the πelectrons are not present in conjugation. Therefore, it is an antiaromatic compound.
Note:
In the presence of fused ring structure, the cycle with maximum number of conjugated π bonds is considered. The lone pairs which are present within the plane only participate in Huckel’s rule, else they are not considered.
