Question
Question: How many sigma and pi bonds do sp, \(s{p^2}\), \(s{p^3}\), \(s{p^3}d\), \(s{p^3}{d^2}\) have?...
How many sigma and pi bonds do sp, sp2, sp3, sp3d, sp3d2 have?
Solution
A single bond formed between the two atoms containing one sigma bond, a double bond formed between the two atoms containing one sigma and one pi bond. A triple bond formed between the two atoms containing one sigma and two pi bonds.
Complete step by step answer:
The property by which the atomic orbitals fuse with each other to form new hybridized orbitals is known as hybridization.
From the hybridization of the central atom, one can know the number of sigma bonds around the central atom.
In acetylene molecules, the sp hybridization is seen which contains two sigma bonds around one carbon atom and two pi bonds around one carbon atom.
The structure of acetylene is shown below.
H−C≡C−H
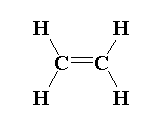
In ethene molecules, sp2 hybridization is seen where three sigma bonds are present around one carbon and one pi bond is present around one carbon atom.
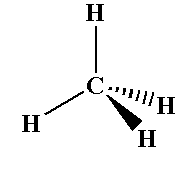
The methane molecule shows sp3 hybridization where four sigma bonds are present around one carbon and no pi bond present.
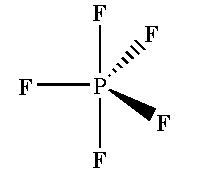
In phosphorus pentafluoride PF5 molecule sp3d hybridization is seen where five sigma bonds are present around one phosphorus atom and no pi bonds are present.
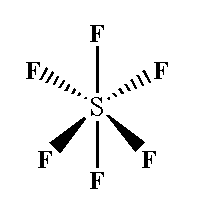
In sulfur hexafluoride SF6, sp3d2 hybridization is seen where six sigma bonds are present around the sulfur atom and no pi bonds are present.
Note:
The number of orbitals taking part in hybridization is the number of sigma bonds made around the central atom. In sp3, sp3d and sp3d2 no pi bond is present as it contains only a single covalent bond.
