Question
Question: How many \(\sigma \) and \(\pi \) bonds are present respectively in the final product obtained by th...
How many σ and π bonds are present respectively in the final product obtained by the Reimer-Tiemann reaction of phenol?
A. 15 and 4
B. 14 and 4
C. 15 and 3
D. 4 and 3
Solution
The covalent bond which is formed by the axial overlapping of the s and p-orbitals are called sigma bonds. The covalent bonds which are formed by the sidewise overlap of the p-orbitals are called pi-bonds.
Complete step by step solution:
- In the question it is asked to find the number of sigma and pi bonds which are present in the product which is formed by the Reimer-Tiemann reaction of phenol.
- Means first we should know the Reimer-Tiemann reaction in detail.
- Phenol reacts with one mole of chloroform in the presence of sodium hydroxide and forms salicylaldehyde as the product.
- The chemical reaction of Riemer-Tiemann reaction is as follows;
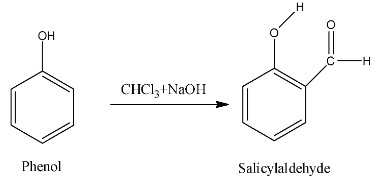
- In the above chemical reaction one mole of phenol is going to react with chloroform and sodium hydroxide and forms salicylaldehyde.
- Now we have to find the number of sigma and number of pi bonds in salicylaldehyde.
- The structure of salicylaldehyde is as follows.:
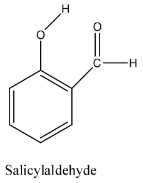
- By seeing the above structure we can say that there are 15 sigma bonds and 4 pi bonds are present in the salicylaldehyde.
So, the correct option is A.
Note: We know that there are 3 pi bonds and 12 sigma bonds are present in the benzene molecule. We have to add the remaining groups attached to the benzene ring to get the number of sigma and pi bonds present in the salicylaldehyde.
