Question
Question: How many sigma and pi bonds are present in tetra cyano methane?...
How many sigma and pi bonds are present in tetra cyano methane?
Solution
The total number of bonds in any compound is the sum of total sigma bonds and pi bonds. The molecular formula of tetra cyano methane is C(CN)4.
Complete step-by-step answer: Tetra cyano methane is also known as carbon tetracyanide. It is a per cyano alkane with molecular formula C(CN)4. The structure can be considered as methane with all hydrogen atoms replaced by cyanide groups. Tetra cyano methane is a solid at room temperature. It decomposes over 160 °C without melting, and although it can be in a dilute vapour, no liquid form is known.
The molecules of tetra cyano methane have a tetrahedral geometry with carbon atoms at the centre and four CN atoms surrounding it. The C-C bond length is 1.484 and the C≡N bond length is 1.161 in the gas form. In the solid the C≡N bond shortens to 1.147. At pressures over 7 GPa tetra cyano methane starts to polymerize to form a disorganised covalent network solid. At higher pressure the colour yellows and darkens to black.
The structure of tetra cyano methane is:
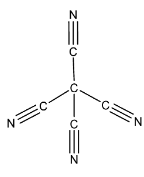
As clearly seen from the structure, there are four C-C sigma bonds and four C-N sigma bonds. So, there are a total of eight sigma bonds. And there are eight C-N pi bonds. So, there are a total of eight pi bonds. Hence, there are a total of 16 bonds in tetra cyano methane.
Note: When asked about the total bonds of C(CN)4, a student can forget to count the bonds between carbon and nitrogen. Then his incorrect answer would come out to be 4.
