Question
Question: How many sigma and pi bonds are present in propene molecules?...
How many sigma and pi bonds are present in propene molecules?
Solution
Here the first thing we get from "propene" usually all bonds between atoms in most of the organic compounds contains one sigma bond each of them. Whenever it is a single bond it contains only sigma bonds. These multiple bonds double and triple however we can understand how it contains sigma and pi bonds. Therefore double bonds have one each and triple bonds have one sigma bond as well as two pi bonds.
Complete step-by-step answer: We all know that propene structural formula is given by CH2=CH−CH3
Propene has 8σ sigma bonds ( C−C bonds and all C−H bonds) and 1π pi bond C1−C2
Whereas
Single Bond = 8σ bond.
Double Bond = 8σ bond + 1π bond.
Triple Bond = 8σ bond + 2π bond.
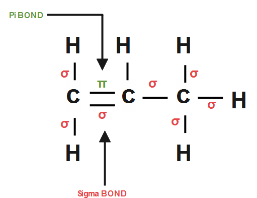
So therefore we have 8σ sigma bond as well as 1π pi bond in a propene molecule.
Note: Note that the π bonding in the orbital is always lower in energy than nonbonding p orbital. Therefore each and every carbon Centre as shown has two electrons in lower energy efficiency and bonding of π orbital the energy of each and every system is being lowered overall and its thus more and more stable, regardless of anion, radical or cation.
Meanwhile the stability of alkyl carbocation is all due to conjugated π electrons. The double bond doesn’t really exist in nature or that environment. Instead of that it is a group of three adjacent, non-hybridized, overlapping p orbitals we call it as a conjugated π electron. We can clearly state that the interaction between all of three p orbitals from three carbon resulting in a really stable cation. It all deprives us of ups and downs to where the location of the electron deficient our carbon atom is.
