Question
Question: How many \[\sigma \] and \[\pi \] – bonds are present in peroxymonosulfuric acid?...
How many σ and π – bonds are present in peroxymonosulfuric acid?
Solution
Hint: So, for finding sigma and pi bonds we have to draw the bond-line structure of peroxymonosulfuric acid and then count each and every bond between any of two atoms or elements.
Complete step by step solution:
Sigma bonds (σ bonds) are the strongest type of covalent chemical bonds. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
We know that Pi bond (πbond) is a bond formed by the overlap of p-orbitals on adjacent atoms, perpendicular to any sigma bond(s) between the same atoms. It is indicated in a Kekule structure or bond-line structure as an extra line parallel to the line which represents the sigma bond.
Chemical formula of peroxymonosulfuric acid is: H2SO5
And structure of peroxymonosulfuric acid:
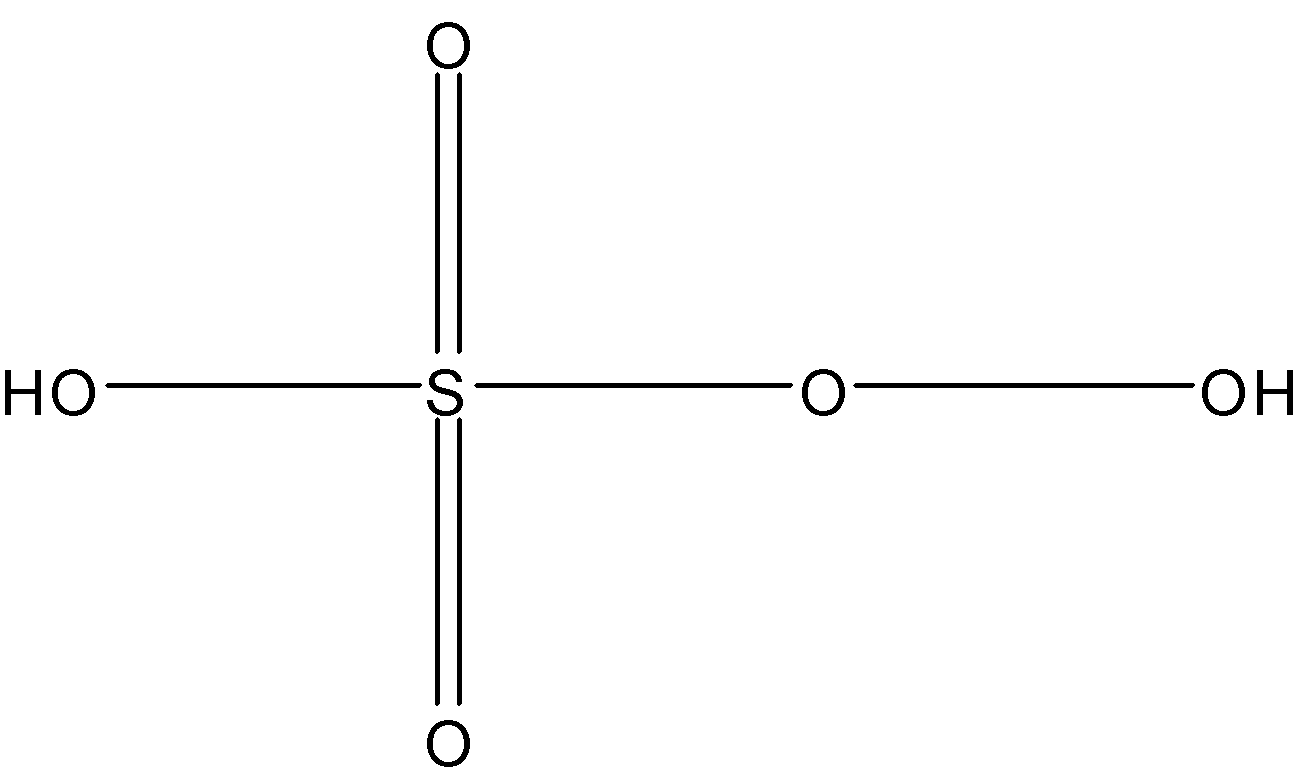
Here, number of sigma bonds = 4 sigma bonds between S and O + 2 sigma bonds between H and O + 1 sigma bond between O and O
= 7
Number of pi bonds = there are two pi bonds between S and O = 2
So, the answer is “7, 2”.
Note: If there is a double or triple bond between any of two atoms then 1st bond we count as sigma bond and other two or one bond we count as pi bond. And in this compound a peroxy bond (O-O).
