Question
Question: How many sigma and pi bonds are in the Lewis structure for \( CHCC{H_2}COOH \) ?...
How many sigma and pi bonds are in the Lewis structure for CHCCH2COOH ?
Solution
In order to answer the question, first we will write the IUPAC name of the given compound and then draw the structural formula of the given compound. And then discuss the presence of sigma and pi bonds.
Complete answer:
The structural formula and IUPAC name of the given compound is
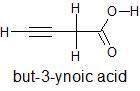
To know the no. of σandπ bonds the Lewis structure of the compound is to be drawn showing all single, double and triple bonds. In this structure
Each single bond is 1σ bond
Each double bond is 1σand1π bond
Each triple bond is 1σand2π bond
Using this information we can get the no. of σandπ bonds in the given structure
7single bonds→7σbonds
1double bond→1σand1πbonds
1triple bond→1σand2πbonds
Note:
3−butenoicacid is a monocarboxylic acid consisting of acetylene carrying a carboxymethyl group. It is a monocarboxylic acid and a terminal acetylenic compound. It is a conjugate acid of a 3−butenoate .
