Question
Question: How many sigma and pi bonds are in \[Se{O_3}^{2 - }\]?...
How many sigma and pi bonds are in SeO32−?
Solution
We need to understand what are sigma and pi bonds and how do we calculate them. In addition, to study about (σ) and (Π) bonds we need to understand molecular orbitals and molecular orbital theory. Understanding of atomic orbitals is also necessary. The first covalent bond between two atoms is always a sigma bond (σ) . Any second or third bond is known as a pi bond. Hence, we would first need to understand the bonding in the given molecule and accordingly calculate the number of sigma and pi bonds in SeO32−.
Complete step by step answer:
The first covalent bond between two atoms is always a sigma bond (σ). Any second or third bond is known as a pi bond. Let us explain this with the help of a simple HCN molecule. This molecule has one single and one triple bond given as follows H−C≡N . As we know that the first covalent bond is always a sigma bond, the single bond between H and C is definitely a sigma bond and the first bond between C and N is a sigma bond and the remaining two are pi bonds.
The given molecule is a selenite ion SeO32− . Its skeletal structure is given below:
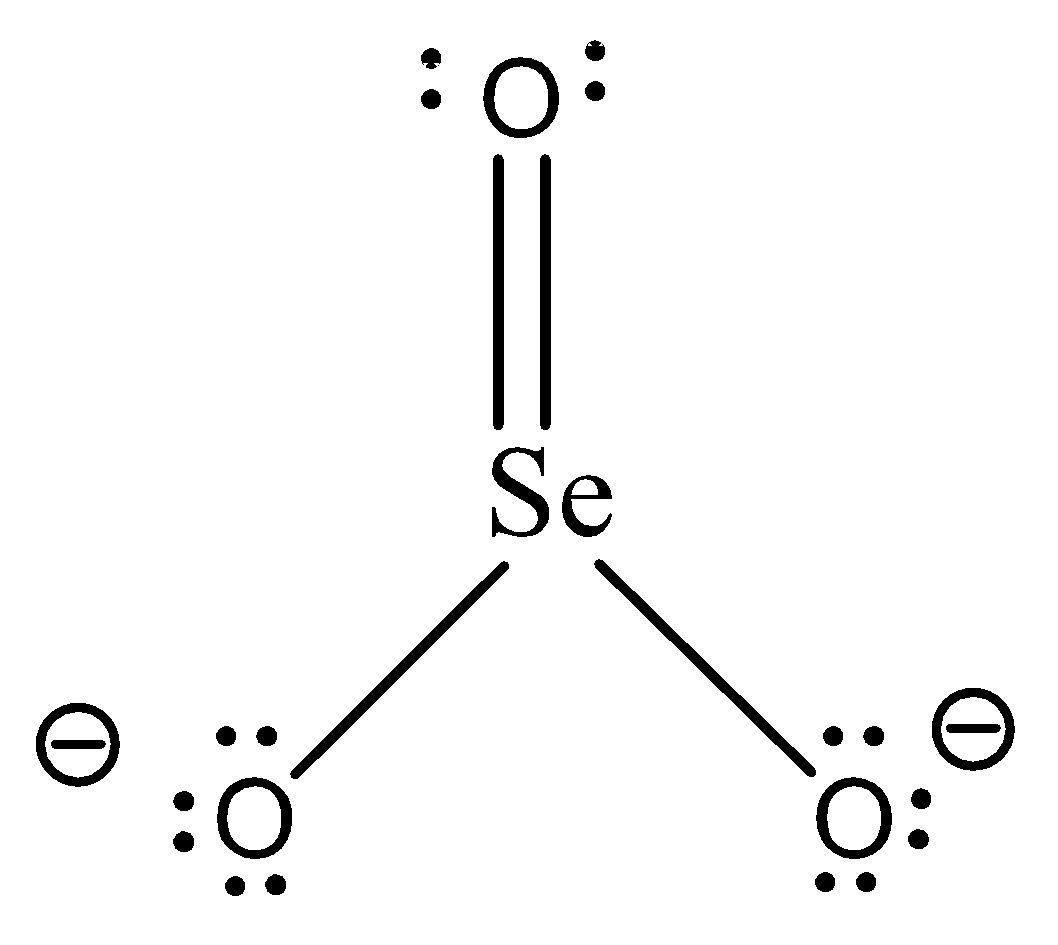
As seen in the skeletal structure, there are 2 single bonds and one double bond. Hence the 2 single bonds are sigma bonds along with the first bond of the double bond and the second bond of the double bond is a pi bond.
Hence, there are three sigma bonds and one pi bond in SeO32− .
Note: It must be noted that there is a simple trick to calculate the number of sigma bonds in a molecule. The formula for this trick is S=x−1 where S− the number of sigma bonds and x is the number of atoms in the molecule. The number of molecules SeO32− is 4. According to the formula, x=4 .Therefore, S=4−1=3 sigma bonds and one pi bond.
