Question
Question: How many sigma and pi bonds are in a 1-propanol molecule?...
How many sigma and pi bonds are in a 1-propanol molecule?
Solution
We will approach this question by drawing an expanded structure of the given compound that is 1-propanol so that we can clearly see every bond through which atoms are connected together. If there is only one bond between 2 atoms then it will be accounted for as a sigma bond and if there is more than 1 bond between 2 atoms even then still one will be counted as sigma bond and the rest are counted as pi bonds.
Complete answer:
Let us first discuss the chemistry of sigma and pi bonds:-
Sigma and pi bonds are generally among the types of covalent bonds which are accordingly distinguished by the type of overlap between 2 atomic orbitals. Covalent bond is observed to be formed by overlapping of atomic orbitals or in simple words, sharing of electron pairs.
-Sigma Bond (σ): These are usually the stiffest and strongest type of covalent bond as they are formed by head-to-head overlapping of atomic orbitals. The electrons that are involved in the formation in sigma bonds are known as sigma electrons and these bonds can exist independently as well as play a role in determining the shape of the molecule.
-Pi Bond (π): These bonds are comparatively weaker than sigma bonds and are usually formed by side-by-side overlapping of atomic orbitals. The electrons that are involved in the formation of pi bonds are known as pi electrons and they cannot exist independently without sigma bonds. Also pi-bonds don’t play any role in determining the shape of molecules.
Generally, if (A) single bond: 1σ bond
(B) Double bond: 1 σ bond, 1π bond
(C) Triple bond: 1 σ bond, 2 π bonds and so on.
Let’s now draw the expanded structure of a 1-propanol molecule so as to clearly see the number of bonds between each and every atom.
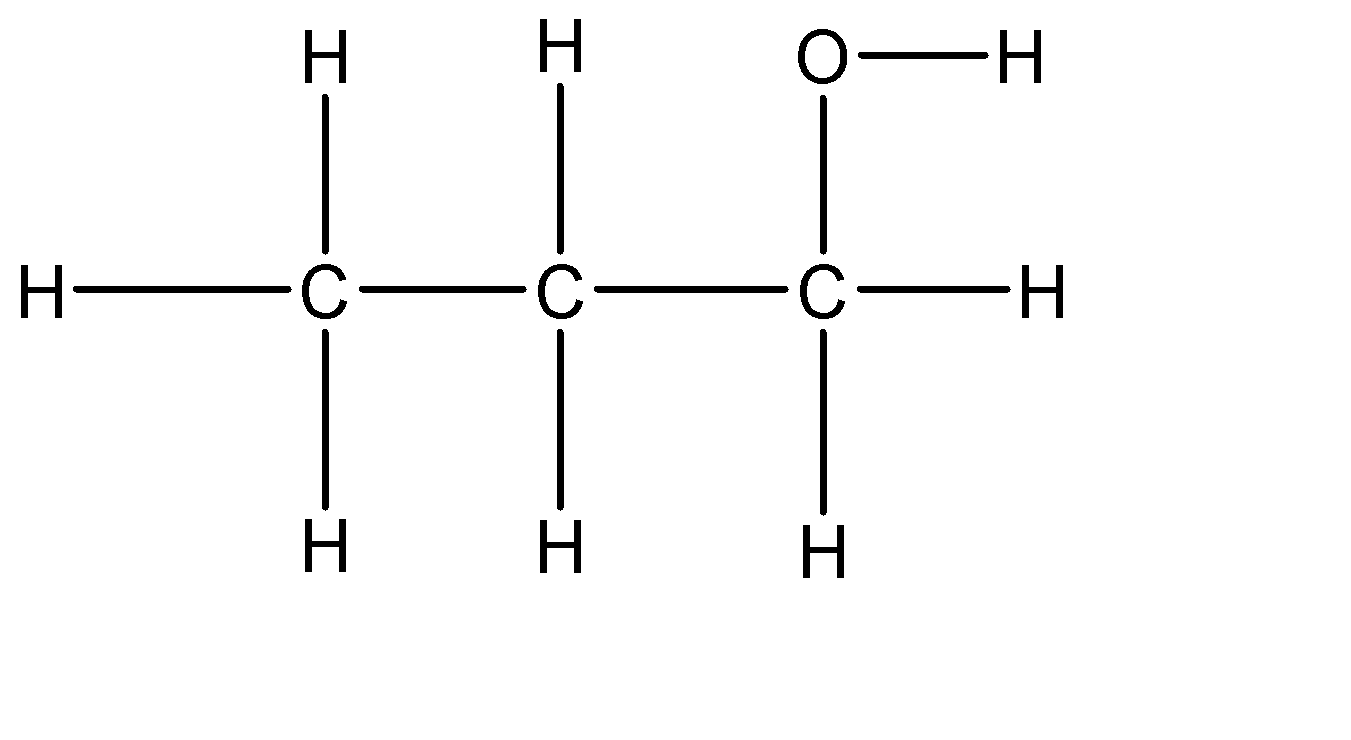
As it can be seen that each atom is connected by at least one single bond which will be counted as σ bonds and no double bonds are present. Therefore the total number of sigma bonds in the given molecule are: 11 and the total number of pi bonds are: 0
Hence there are 11 sigma (σ) bonds and 0 pi (π) bond in 1-propanol molecule.
Note:
-If question asks only about the number of sigma bonds then an alternative method can be used without making the complete structure of the compound (refer only for carbon containing compounds) by the given formula:-
No. of sigma bonds= [(No. of carbon atom)-1] + [Total no. of other atoms] in the given compound.
For the above compound:-
Total no. of C atom = 3 Total no. of other atoms = 9
No. of sigma bonds = (3-1) + 9 = 11
